The Association between Resting Heart Rate and Arterial Stiffness in Men
Article information
Abstract
Background
Early detection of vascular change may improve prediction of subclinical stage of cardiovascular disease, allowing intervention to prevent overt vascular damage. High heart rate is known to increase cardiovascular morbidity and mortality rate in the general population and in individuals with cardiovascular disease. We aimed to investigate the association between resting heart rate (RHR) measured using electrocardiogram (ECG) and arterial stiffness measured using the cardio-ankle vascular index (CAVI) in men.
Methods
Data were collected from 5,629 men aged between 20 and 78 years who visited a single-site health promotion center. RHR was measured in a supine posture after resting for 10 minutes using an ECG. Arterial stiffness was measured using the CAVI. The cutoff value for high CAVI was ≥9.0.
Results
RHR was one of the major determinants of high CAVI after adjusting for age, waist circumference, mean arterial pressure, glycosylated hemoglobin level, triglyceride level, white blood cell count, and lifestyle factors. When RHR groups were defined according to the RHR quartiles, the odds ratio of group with RHR ≥70 bpm, for high CAVI was 3.62 (95% confidence interval [CI], 2.21–5.91) after adjusting for age and lifestyle factors. This association was not changed after adjusting for all other covariates (odds ratio, 2.39; 95% CI, 1.36–4.19).
Conclusions
RHR measured using ECG is significantly associated with arterial stiffness in men not taking medications for hypertension, dyslipidemia, or diabetes. These findings suggest that RHR may be useful in assessing cardiovascular risk in men.
INTRODUCTION
Cardiovascular disease is the number one cause of death worldwide, estimated to cause more than 17 million deaths annually.1) The overall prevalence of clinical atherosclerotic cardiovascular disease in Korea reaches 101 per 1,000 individuals in 2015.2) Risk management and early detection are important for primary prevention and for reducing the burden associated with morbidity and mortality from cardiovascular disease.
Heart rate is known to increase cardiovascular morbidity and mortality rate in healthy people as well as those with hypertension, diabetes, or coronary artery disease.3456) Heart rate has been also associated with incident cardiovascular disease risk factors, such as hypertension, diabetes, and obesity because elevated heart rate is related to imbalance of the autonomic nervous system, which can affect blood pressure and glucose and lipid metabolism.7) The resting heart rate (RHR), measured using electrocardiogram (ECG), is highly reproducible and routinely performed in many clinical settings.
Many studies have demonstrated that presence of arterial stiffness predicts adverse cardiovascular outcomes in certain populations.8910) Although pulse wave velocity (PWV) is widely used for assessment of arterial stiffness, it has the limitation of being affected by blood pressure during the measurement.11) Unlike PWV, the cardio-ankle vascular index (CAVI) reflects smooth muscle contraction rather than changes in blood pressure; therefore, it can assess arterial stiffness by reflecting vascular tone regardless of blood pressure. Moreover, it reflects not only structural stiffness, but also functional stiffness caused by smooth muscle contraction.12) Early detection of vascular change may improve prediction of a subclinical stage of cardiovascular disease; with intervention, progression to overt vascular damage can be prevented. There have been studies investigating the relationship between heart rate and arterial stiffness, but in most studies, PWV has been used and few studies have been conducted on subjects who do not take medications that can affect arterial stiffness. The aim of study was to investigate the association between RHR and arterial stiffness in men that measured using the CAVI.
METHODS
1. Study population
Data was gathered from 7,842 men aged 20 to 78 years who had visited a single-site health promotion center for periodic health examination between January 2013 and December 2017. Subjects who met one of the following conditions were excluded: omissions in anthropometric measurements or laboratory tests (n=276); ankle-brachial index (ABI) <0.9 or ≥1.4 (n=24); thyroid-stimulating hormone <0.55 µIU/mL (n=289); a history of cerebrovascular- or cardiovascular disease (n=240); or a history of taking medications that could affect CAVI (n=1,394). After the criteria were applied, data from 5,629 men were analyzed. The study was approved by the Institutional Review Board of the Eulji University Hospital, Daejon, Korea.
2. Data collection and anthropometric measurements
All subjects completed a self-reported questionnaire regarding lifestyle related information. Detailed medical history including current medication uses was collected through medical interview. Alcohol consumption was defined as more than 5 drinks per day or more than 15 drinks per week according to the National Institute on Alcohol Abuse and Alcoholism criteria.13) A smoker was defined as an ex-smoker who had stopped within the last 6 months or current smoker. Regular exercise was defined as high-intensity exercise for more than 60 minutes per week, or low- and medium-intensity exercise for over 150 minutes per week.
Height and body weight were measured using a body composition analyzer (Inbody BSM 720; Biospace, Seoul, Korea) with shoes off and wearing a light robe. Waist circumference was measured midway between the lowest rib and the iliac crest in a standing position. Body mass index was calculated as the body weight divided by the height squared (kg/m2).
Blood pressure was measured using an automatic device (EASY X 800 R; Jawon Medical, Seoul, Korea) in a sitting position after resting for 10 minutes. The RHR was measured in a supine posture using an electrocardiogram (PageWriter TC30 Cardiograph; Philips Medical System, Andover, MA, USA) after resting for 10 minutes. Mean arterial pressure (MAP) was calculated as: [diastolic blood pressure (mmHg) + {systolic blood pressure (mmHg) - diastolic blood pressure (mmHg)} / 3].
3. Laboratory measurements
Blood samples were collected after an overnight fast lasting at least 12 hours and analyzed within 3 hours after collection. Complete blood counts were measured using an ADVIA 2120i (Siemens Healthcare Diagnostics, Deerfield, IL, USA). Blood chemistry was measured with an enzymatic technique using a Chemistry XPT (Siemens Healthcare Diagnostics). Direct methods were used to measure the low-density lipoprotein cholesterol. Glycosylated hemoglobin (HbA1c) was measured with ion-exchange high-performance liquid chromatography using an HLC-723 G7 instrument (Tosoh Corp., Tokyo, Japan) calibrated to the diabetes care and complications test standard.
4. Cardio-ankle vascular index measurement
CAVI was measured using a vascular screening system (Vasera VS-1000; Fukuda Denshi, Tokyo, Japan). Subjects were examined in a supine position after 10 minutes of bed rest. Cuffs were applied to both arms and ankles. Electrodes were attached to both arms. A microphone for phonocardiography was placed on the sternum. Pressures and waveforms of the brachial and ankle arteries and ECG signals were measured. Carotid-ankle PWV and CAVI were automatically calculated using the vascular screening system. CAVI was calculated according to the following equation: CAVI = a {(2ρ / ΔP) × ln (Ps / Pd) PWV2} + b. Where Ps and Pd are systolic and diastolic blood pressure, respectively; PWV is pulse wave velocity between the aortic valve and ankle; ΔP is Ps − Pd; ρ is blood density; and a and b are constants. In this study, the higher value of the right or left CAVI was used for the analysis. A high-CAVI was defined as ≥9.0.
5. Statistical analysis
Subjects were divided into two groups according to CAVI as follows: normal-CAVI group (CAVI <9.0) and high-CAVI group (CAVI ≥9.0). RHR groups were defined according to the RHR quartiles as follows: RHR ≤57 bpm (group 1); 57 bpm < RHR ≤63 bpm (group 2); 63 bpm < RHR ≤69 bpm (group 3) and; ≥70 bpm (group 4). To compare the general characteristics between normal- and high-CAVI groups, Student's t tests and chi-square tests were utilized for continuous and categorical variables, respectively. The partial correlation coefficients adjusted for age were used to evaluate the relationship between CAVI and other variables. Multivariate linear regression analysis was performed to identify the effect of the determinants on the CAVI. In the model, the independent variables were those that had a significant relationship with the CAVI in the partial correlation analysis. After subjects were divided into four groups according to the quartiles of the RHR, logistic regression analyses were used to estimate the odds ratios (ORs) for high CAVI in each group. All statistical analyses were performed using SPSS version 25.0 software (IBM, Chicago, IL, USA). A probability value of P<0.05 was considered significant.
RESULTS
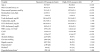
The mean age of the 5,626 subjects was 49.0±6.6 years. The mean CAVI of all subjects was 7.16±0.84. One hundred sixty-five subjects (2.9%) belonged to the high-CAVI group. The mean CAVI were 7.09±0.73 and 9.57±0.58 in normal- and high-CAVI group, respectively (P<0.001). The mean RHRs were 63.5±9.1 bpm and 67.0±10.5 bpm in the normal- and high-CAVI groups, respectively. Age, MAP, HbA1c, white blood cell (WBC) counts, ABI, hypertension, and diabetes were significantly higher in high-CAVI group than normal-CAVI group. More subjects in the normal-CAVI group had a history of alcohol consumption (P<0.05). The general characteristics of the subjects in normal- and high-CAVI groups are shown in Table 1.
CAVI had a positive correlation with MAP, HbA1c, triglyceride, WBC count, and RHR after adjusting for age (P<0.05). In contrast, waist circumference was negatively correlated with CAVI. Table 2 shows that RHR is one of the major determinants of CAVI when age, waist circumference, MAP, HbA1c, triglyceride and WBC count, alcohol intake, current smoking, and regular exercise were included in the regression model. Moreover, age, waist circumference, MAP, HbA1c, triglyceride, and lifestyle factors were significantly associated with CAVI and accounted for up to 26.2% of the CAVI.
Table 3 shows the ORs for high CAVI in each group of RHR after adjustment for age and lifestyle factors. The adjusted OR of group 4 (RHR ≥70 bpm) for high CAVI were 3.62 (95% CI, 2.21–5.91) after adjusting for age and lifestyle factors. This association was significant after adjusting for all other covariates (OR, 2.39; 95% CI, 1.36–4.19).
DISCUSSION
In this study, RHR showed a significant association with arteriosclerosis in men, as measured using CAVI. According to the study design, only men with no history of taking medications that could affect CAVI were included for analysis. In addition, after adjusting for age, lifestyle factors, and all other covariates, the odds for high CAVI in men with RHR ≥70 bpm were significantly increased.
RHR has been directly related to all-cause mortality, cardiovascular mortality, and development of clinically evident cardiovascular disease in the general population and patients with hypertension or coronary artery disease.345) High heart rate has been also associated with incident cardiovascular risk factors, such as hypertension, diabetes, and obesity.7) However, heart rate measurements are rarely used in clinical practice to predict cardiovascular risk because they are affected by physical and environmental conditions, psychological stimuli, and measurement methods.14) Additionally, there is no standard method to measure heart rate, which can lead to inconsistent results. One study showed that the reproducibility of heart rate measurement was particularly poor when it was above the level of 85 bpm due to high variability.15) Conversely, RHR measurement using ECG is the most precise method of heart rate measurement and is routinely carried out in many clinical settings. It is not known whether high measurement accuracy leads to results that are more meaningful.16)
Decreased arterial compliance is one of the earliest detectable signs of structural and functional adverse changes within the vessel wall. CAVI is an index for the overall stiffness of the artery from the aorta to the ankle and is highly reproducible compared to the aortic- or brachial-ankle PWV because it is not affected by blood pressure.17) In this study, the cutoff value for high CAVI was defined as greater than 9.0 because the reported cutoff value for the presence of coronary stenosis is 8.91.18)
In a multi-ethnic study of atherosclerosis study, RHR measured using ECG had a direct relationship with arterial stiffness measured using an imaging modality for carotid- and aortic distensibility in an ethnically diverse population free of known cardiovascular disease.19) Two studies have reported an association between heart rate and arterial stiffness in Korean adults.2021) In these studies, heart rate was measured using ECG or an automated blood pressure device in the supine position. In a study of normotensive Korean Americans, higher RHR was independently associated with increased arterial stiffness measured using carotid-femoral PWV.20) However, the number of subjects was small, and other variables such as biochemical markers and lifestyle factors were not included in the analysis. Another study reported similar results of an association between RHR and brachial-ankle PWV (baPWV) after adjusting for the presence of drugs that could modify both RHR and PWV such as antihypertensive, antidiabetic and lipid-lowering medications.21) However, the authors did not verify whether RHR was a determinant of baPWV. In our study, subjects who received medication for hypertension, dyslipidemia, or diabetes were excluded because these medications may affect RHR and CAVI. Because arterial stiffness depends on prevailing blood pressure, antihypertensive treatment is expected to reduce it in proportion to the blood pressure reduction. In addition, antihypertensive medications might differ in their effects on the structure and function of the arterial wall. Meanwhile, in a meta-analysis of randomized clinical trials, short-term statin therapy was found to have beneficial effects on arterial stiffness.22) Because these medications may affect vascular compliance or heart rates during short- and long-term treatment, subjects who were taking medications for hypertension or dyslipidemia were excluded. We also excluded patients who were receiving antidiabetic medications because most patients were also receiving statins.
Heart rate is correlated with serum catecholamine that could affect lipid and glucose metabolism. In this study, RHR was significantly correlated with obesity indices, blood pressure, glucose indices, lipid profile, and inflammatory indices after adjusting for age. This finding is similar to that of a previous study that reported a positive association between heart rate and hypertension, diabetes, and obesity.23) These findings suggest that heart rate elevates the risk of arteriosclerosis by affecting conventional risk factors for cardiovascular disease. Mechanical or chemical stresses, such as hypertension, inflammation, and glycation end-products, induce structural changes within the vascular wall and extracellular matrix (atherosclerosis). During this process, collagen deposition and calcification increase in the vessel wall, leading to increased vascular stiffness and decreased compliance (arteriosclerosis). Increased vascular stiffness results in elevated systolic blood pressure, decreased aortic reservoir and buffering, and results in adverse cardiovascular outcomes.24) Several studies have reported that the association between heart rate and cardiovascular mortality was observed only in men, but not in women, and differences in heart rate between sexes have also been reported.25262728) During preliminary analysis for the present study, we also found that CAVI was not correlated with RHR in women. Messerli et al.26) reported that women had a higher RHR and pulse pressure than men and this difference was related to greater cardiac output with low total peripheral resistance. The authors concluded that for any level of blood pressure, the risk of hypertensive cardiovascular disease was lower in women than in men.26)
Our study had several limitations. First, we could not verify causality between RHR and arterial stiffness because of the nature of the cross-sectional design. Second, because the study subjects were from a single site, there is a limitation in generalizing the results of this study. Third, only one measurement of RHR may not represent the individual's baseline RHR.
In conclusion, RHR measured using ECG is significantly associated with arterial stiffness in men who were not taking medications for hypertension, dyslipidemia, or diabetes. These findings suggest that RHR measured using ECG may be useful in assessing cardiovascular risk in individuals.
ACKNOWLEDGMENTS
We would like to thank Editage (www.editage.co.kr) for English language editing.