| Korean J Health Promot > Volume 19(3); 2019 > Article |
|
ABSTRACT
Background
Methods
Results
REFERENCES
Table┬Ā1
General characteristics of the subjects between normal- and high-CAVI groupsa
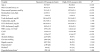
Values are presented as the mean┬▒standard deviation for continuous variables and number (%) for categorical variables.
Abbreviations: ABI, ankle-branchial index; CAVI, cardio-ankle vascular index; HbA1c, glycosylated hemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein; WBC, white blood cell.
aGroup 1 is defined as CAVI <9.0; group 2 is defined as CAVI Ōēź9.0.
bP value using Student's t-test for continuous variables and chi-square test for categorical variables.
cHypertension was defined as systolic blood pressure Ōēź140 mmHg or diastolic blood pressure Ōēź90 mmHg.
dDiabetes was defined as glycosylated hemoglobin Ōēź6.5% or fasting plasma glucose concentration Ōēź126 mg/dL.
eHypercholesterolemia was defined as LDL-cholesterol Ōēź160 mg/dL.
Table┬Ā3
Adjusted odds ratios for high-CAVI (Ōēź9.0) according to the resting heart rates groupsa

Abbreviation: CAVI, cardio-ankle vascular index.
aGroup 1 is defined as resting heart rate Ōēż57 bpm (n=1,451); group 2 is defined as 58ŌĆō63 bpm (n=1,538); group 3 is defined as 64ŌĆō69 bpm (n=1,355); group 4 is defined as Ōēź70 bpm (n=1,285).
bAdjusted for age.
cAdjusted for age, alcohol drinking, current smoking, and regular exercise.
dAdjusted for age, waist circumference, mean arterial pressure, glycosylated hemoglobin, triglyceride, white blood cell count, alcohol drinking, current smoking, and regular exercise.
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 1,027 View
- 2 Download
- Related articles
-
The Association between Metabolic Syndrome and Intraocular Pressure2011 June;11(2)
The Association Between Eating Frequency and Metabolic Syndrome2011 March;11(1)
Association between Serum Carotenoids and Metabolic syndrome2009 December;9(4)





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print