Evaluating the Accuracy and Clinical Utility of Bioelectrical Impedance Analysis (BIA) Devices for Body Composition Measurements in Clinical Practice: Comparison of Four Types of BIA Equipment and Dual-Energy X-ray Absorptiometry
-
So Youn LEE, MD1
 , Hwayeon SUN, PhD2
, Hwayeon SUN, PhD2 , Sung Hwan BAE, MD3
, Sung Hwan BAE, MD3 , Ju Yeon SON4
, Ju Yeon SON4 , Byungwook YOO, MD, PhD2
, Byungwook YOO, MD, PhD2
- Received April 18, 2025 Revised May 17, 2025 Accepted May 18, 2025
- ABSTRACT
-
- Background
- This study aimed to evaluate whether Bioelectrical Impedance Analysis (BIA) can serve as a practical alternative to Dual-Energy X-ray Absorptiometry (DEXA) for measuring body composition in clinical settings.
- Methods
- BIA (InBody970, InBody770, InBody770C, and InBody270C) and DEXA were repeatedly used for measurements on the same day (n=100). Participants fasted for at least 4 hours and stood upright for 10 minutes before the measurements to stabilize body water composition.
- Results
- All four BIA devices (InBody970, InBody770, InBody770C, and InBody270C) showed a high correlation with DEXA, with Pearson correlation coefficients (r) of ≥0.97 for both muscle mass and body fat mass. Among them, the InBody770C demonstrated a slightly higher correlation in body fat mass measurements (P<0.05). However, Lin’s Concordance Correlation Coefficient ranged from 0.939 to 0.941, indicating only moderate agreement. In addition, Bland–Altman analysis revealed statistically significant mean biases and wide limits of agreement across all devices, suggesting that strong correlation does not necessarily imply measurement equivalence.
- Conclusions
- Given the high correlation between BIA and DEXA, BIA may serve as a useful tool for tracking body composition. However, moderate agreement levels suggest that caution is warranted when interpreting BIA as a substitute for DEXA in clinical practice.
- INTRODUCTION
- INTRODUCTION
Bioelectrical Impedance Analysis (BIA), a non-invasive and widely used method, estimates body composition by measuring electrical conductivity [1]. Because excess body fat is linked to health risks, accurate assessment of fat and fat-free mass (FFM) is essential [2].BIA relies on calibration equations based on reference equipment, such as Dual-Energy X-ray Absorptiometry (DEXA) and magnetic resonance imaging (MRI), rather than directly measuring specific body components [3]. Since repeated measurements of fat mass (FM) and FFM are often required in patients who have undergone surgery [4], BIA is particularly useful for patients needing repeated body composition monitoring [5].Body composition analysis serves as a valuable diagnostic tool for assessing overall health and provides insight into nutrition, obesity, and muscle loss across diverse populations [2].Both BIA and DEXA have distinct strengths and limitations. BIA is easy to use, non-invasive, radiation-free, and well-suited for repeated measurements in clinical and research settings. It provides quick results with minimal patient burden [3].However, BIA’s accuracy can be influenced by factors such as body shape, hydration, ethnicity, age, and fat level. It also requires pre-measurement preparation—e.g., fasting for 4 hours and avoiding exercise for 8 hours prior.On the other hand, DEXA provides more precise estimates with an error margin of 1%–2% and minimal radiation exposure (0.001 mSv per scan) [6]. While preparation is minimal, DEXA machines are costly, non-portable, and require patients to remain still in a fixed position for several minutes. Additionally, the limited table size and standing requirements may not accommodate obese patients or those unable to stand [5].As our understanding of obesity evolves, it is clear that body mass index (BMI), waist circumference, and height alone are insufficient. Emerging categories such as lean obesity, sarcopenic obesity, and metabolically healthy obesity highlight the need for more comprehensive clinical indicators. Most studies comparing BIA and DEXA have been conducted in Europe [7,8], limiting applicability to Asian populations. Furthermore, research directly comparing multiple BIA models, especially newer ones like InBody990, remains scarce.This study aims to assess whether BIA can serve as a reliable alternative to DEXA for clinical body composition analysis, focusing particularly on FFM estimation and sarcopenia evaluation. With advancements in BIA technology—such as expanded frequency ranges and improved prediction algorithms—its potential clinical value continues to grow. Previous research compared two-lead BIA with MRI, leading to the development of prediction equations for estimating whole-body skeletal mass [9]. This study adopts the stepwise selection method with the Schwarz Bayesian Information Criterion (SBC) for BIA prediction modeling [10].
- METHODS
- METHODS
- Participants and study period
- Participants and study period
A total of 100 healthy adults (42 males, 58 females) over the age of 20 who visited the Department of Family Medicine, Soonchunhyang University Hospital in December 2020, were able to stand unaided for 5 minutes, and had no history of disease or medication use were included in this experiment. Individuals with metallic implants, limb deformities, or pregnancy were excluded.All participants provided written informed consent. This study was approved by the Institutional Review Board (IRB) of Soonchunhyang University Hospital (IRB no. 2020-09-029) and complied with the principles of the Declaration of Helsinki and its contemporary amendments.- Bioelectrical Impedance Analysis evaluation
- Bioelectrical Impedance Analysis evaluation
Professional body composition analyzers (InBody970, InBody770, InBody770C, and InBody270C; Biospace) were used. Each analyzer was equipped with eight tactile electrodes and operated at multiple frequencies (1, 5, 50, 250, 500, and 1,000 kHz). BIA and DEXA measurements were conducted on the same day. After having fasted for at least 4 hours, the subjects wore light clothing, removed all metal components from their bodies, and rested in a standing position for at least 10 minutes in order to stabilize body water before proceeding with the tests.The arms were stretched out so that the armpits were not constricted, and knee length shorts were worn to prevent the thighs from sticking together. All four fingers were positioned so as to touch the electrode surface of the measurement device, with the thumb staying in contact with the oval part. The heels were aligned with the tips of the feet electrodes, on which participants stood barefoot on the foot electrodes and held the hand electrodes with both hands. For accurate measurements and smooth current flow, the hands and feet were moistened with an electrolyte tissue before the measurements. The time between measurements on each piece of equipment was never more than 5 minutes, and all measurements were taken twice.Equivalence of test values between measuring chambers and equipment is secured using a human resistance zig (RCzig). Next, the calibration unit is connected to the equipment. The eight electrodes of the calibration unit are connected to four hand electrodes (2 on the right and left) and four power electrodes (2 on the right and left sides) using electrolyte tape. In the quality control report, the precision of impedance (Z) and resistance (R) is less than ±1% of the extremities, and if it is less than ±3% of the body, it is considered a pass. The human body resistance model zig's accuracy can be confirmed with a Digit Multimeter (Agilent) or Digital Phosphor Oscilloscope (Tektronix).Body composition results are using proprietary prediction algorithms that are embedded in the device’s firmware.- Dual-Energy X-ray Absorptiometry evaluation
- Dual-Energy X-ray Absorptiometry evaluation
DEXA measurements (Hologic Inc.) were conducted immediately following BIA testing, with a maximum interval of 10 minutes between assessments. Participants lay in a supine position for a full-body scan assessing muscle mass, FM, and bone mineral content. Participants lay in a supine position and were scanned muscle mass, body FM, total FM and ratio, and the body FM ratio. The time spent on these measurements was approximately 10 minutes. DEXA outputs included FFM, FM, body fat percentage, and bone mineral content, calculated using standard software algorithms.- Schwarz Bayesian Information Criterion optimization
- Schwarz Bayesian Information Criterion optimization
- Analysis methods
- Analysis methods
Data were expressed as mean±standard deviation or number (%). A chi-square test and correlation analysis were performed on muscle mass (FFM) and body FM with BIA and DEXA. Also, the mass value measured by DEXA was assumed to be the gold standard [12]. The Bland–Altman plot [13] shows random errors and systematic bias between DEXA and BIA measurements. Also, to confirm the strength agreement between DEXA and BIA machines, Lin’s Concordance Correlation Coefficient (CCC) [14] was calculated, and was found to be suitable for evaluating strength agreement between two methods, and Lin’s CCC is an evaluation method used frequently in clinical practice to confirm new methods for gold standards measurement [15]. Since it is a product of the squared difference between the measurements and the Pearson Correlation Coefficient, a high coefficient means both strong agreement and linear relationship between the two methods. A strength of agreement value of <0.90 is interpreted as poor, 0.90–0.95 is moderate, 0.95–0.99 substantial, and 0.99 or greater is almost perfect. This was done according to McBride’s suggestion [16]. All collected data were analyzed using SPSS PASW Statistics ver. 27.0 (IBM Corp.) and R studio ver. 4.1.3 (Posit Software). The statistical significance level was set to 0.05 or less.
- RESULTS
- RESULTS
- General characteristics of study subjects
- General characteristics of study subjects
Among the 100 subjects, 42 males and 58 females had an average age of 41.3±11.3 years. There was a significant age difference between males and females (39.0±10.8 years for males and 43.0±11.3 years for females) (P=0.01).The average weight of the subjects was 65.8±13.6 kg (males: 76.4±11.5 kg; females: 58.1±9.2 kg), and the average height was 166.1±9.1 cm (males: 174.9±5.7 cm; females: 159.8±5.0 cm). The average BMI was 23.7±3.5 kg/m2 (males: 24.9±3.0 kg/m2; females: 22.8±3.6 kg/m2), as shown in Table 1.- Analysis results
- Analysis results
Existing devices such as the InBody970 and InBody770 demonstrated very high correlations with DEXA, with r ≥0.97 in the measurement of both muscle mass and body FM, as shown in Table 2. These results were consistent across a wide range of participant characteristics, including various BMIs, ages, and specific body types such as muscular or heavier-set individuals.In particular, the InBody770C showed a similarly high correlation with DEXA, with r ≥0.97 for both muscle mass and body FM measurements.In particular, in terms of body FM, the correlation is slightly higher than that of the existing equipment, the InBody970 and InBody770 (Fig. 1).The InBody770C showed no difference in the precision or performance of its measured values compared to other existing devices. Similarly, the InBody270C demonstrated a very high correlation with DEXA (r≥0.97) for both muscle mass and body FM. Notably, the correlations observed for the InBody970 and InBody770 were slightly higher than those of other devices (Fig. 2).BIA Prediction Equations and Cross-Validation Statistics by Variable used in Table 3 for FFM is a SBC optimization method. However, the SBC affects the complexity of the model negatively.The CCC for the prediction equation results compared to DEXA was 0.940 (95% confidence interval [95% CI]: 0.926–0.952) for InBody970, 0.941 (95% CI: 0.927–0.952) for InBody770, 0.939 (95% CI: 0.925–0.951) for InBody770C, and 0.940 (95% CI: 0.926–0.951) for InBody270C. According to McBride [16], these values indicate a moderate strength of agreement. The intraclass correlation coefficient values were nearly identical to the CCC values, supporting consistency across measurements.Finally, FFM was compared using Bland–Altman plots between the four BIA devices and DEXA. In the plots, the difference between the two measurements (DEXA and BIA-predicted FFM) was plotted against their average to identify any systematic bias or trends based on measurement magnitude.Despite using prediction equations, Bland–Altman plots revealed wide limits of agreement across all devices. The mean biases were statistically significant for all four devices: InBody970 (–2.89; 95% CI: –6.27 to 0.50), InBody770 (–2.89; 95% CI: –6.14 to 0.37), InBody770C (–2.94; 95% CI: –6.15 to 0.26), and InBody270C (–2.94; 95% CI: –6.10 to 0.22) (Fig. 3).
- DISCUSSION
- DISCUSSION
- Conclusions
- Conclusions
This study confirmed that BIA devices show high correlation with DEXA, but significant variability in FFM estimates was observed via Lin’s CCC and Bland–Altman analysis. These findings indicate that while BIA is not interchangeable with DEXA, it may still be useful for sarcopenia screening due to its convenience and cost-effectiveness.Advances such as high-performance System on a Chip have improved measurement accuracy and speed by refining impedance algorithms. Still, variations in device algorithms challenge measurement consistency, highlighting the need to understand each device’s limitations.Despite progress, BIA is not suitable for precise diagnosis but remains valuable for tracking nutritional status and identifying individuals with muscle weakness.
Both InBody770C and 270C showed high correlation with DEXA (r≥0.97). However, Bland–Altman plots revealed wide limits of agreement, indicating that strong correlation does not necessarily mean good agreement—highlighting methodological differences. Devices using different bioelectrical impedance technologies are generally more accessible and economical, but the underlying estimation methods differ. Results from Lin’s CCC in Table 3 and the Bland–Altman plot in Fig. 3 illustrate the fundamental differences in measurement principles between BIA and DEXA, reaffirming DEXA’s role as the gold standard.Until now, DEXA has been considered the gold standard in terms of reproducibility and accuracy among body composition testing methods used in clinical practice.DEXA accurately distinguishes fat, soft tissue, and bone density by measuring differences in radiation absorption [17]. In terms of results, the reliability of such a method is high, but no matter how small the amount, users experience radiation exposure via this measurement method. However, DEXA accuracy may be reduced in patients with morbid obesity.Despite being accurate, DEXA has drawbacks such as radiation exposure, high cost, and the need for a fixed posture, limiting its use in some clinical settings and with non-ambulatory patients. Popular systems like Hologic Horizon W and GE Lunar Prodigy are also expensive and not portable. A Korean study found that while DEXA devices correlate well for muscle mass, they differ significantly in fat and lean mass estimates due to algorithmic variations [18]. Additionally, several studies have highlighted discrepancies between different DXA technologies and models. Tothill et al. [19] compared pencil-beam and fan-beam systems, revealing systematic differences in total body composition measurements. Watson et al. [20] further demonstrated significant variations in bone density and body composition between two GE Lunar systems. Kaminsky et al. [21] also reported that the precision of regional fat mass estimates can vary depending on the DXA equipment used.In contrast, BIA offers quick, portable measurements. High-frequency BIA (e.g., InBody970, BWA) improves fluid analysis [22], but often overestimates fat-free mass and underestimates appendicular lean mass and percent body fat due to body composition and demographic variability [23,24].Despite this, high-frequency bioelectrical impedance analysis is gaining traction as a cost-effective, radiation-free alternative for sarcopenia screening [24]. Multiple studies from Taiwan, Japan, Korea, and China confirm BIA’s high correlation with DEXA, though muscle mass may be under- or overestimated depending on BMI and model used [25-29].Older BIA models like the InBodyS10 showed low agreement with DEXA, as expected [30], and BIA consistently underestimated skeletal mass compared to MRI in Janssen et al.’s study [9].While BIA is less precise, its potential for monitoring lean and FM makes it useful in managing metabolic diseases and conditions like sarcopenia. In addition, when cross-validating Asian and Caucasian subjects, it was found that the BIA equation had a margin of error of approximately 5% for Caucasians, African-Americans, and Hispanics, but around 10% for Asian populations [9]. This suggests that to ensure diagnostic accuracy in clinical settings, predictive equations must be tailored to genetic and ecological differences. Therefore, this study employed the SBC method to optimize prediction. Nevertheless, results showed that BIA remains considerably limited in replacing DEXA.Consistent with previous studies, BIA underestimated muscle mass compared to DEXA, but trend monitoring remains clinically useful. Concordance between InBody970 and whole-body DEXA supports BIA use, especially with conversion formulas [31].Sarcopenia, officially recognized by global health authorities, contributes to rising healthcare costs and is linked to conditions that increase the risk of falls and disability [30]. Sarcopenia has been discussed as a clinical condition of concern by global health organizations [32]. In the United States, it was officially classified as a disease in 2016 under the ICD-10-CM code M62.84 by the CDC [33], and in Korea, it has been recognized as a disease entity since 2021, with a reported prevalence of 13.1%–14.9% in elderly men and 11.4% in women [34]. Its severity is higher in patients with cerebral disease. Given BIA’s high correlation with DEXA, it may be useful for tracking muscle loss in older adults.This study’s strength lies in its inclusion of participants with diverse age and BMI. It also compared three BIA devices—InBody270C (20–100 kHz), InBody770 (up to 1 MHz), and InBody990 (2–3 MHz)—showing improved accuracy and reproducibility with newer models. InBody990 also supports supine measurements, suggesting future studies in bedridden or disabled patients. However, it remains uncertain whether BIA is sufficiently accurate for use in critically ill patients. If the accuracy of body water measurement can be validated, BIA may be employed in various clinical settings [35].This study has several limitations. First, it did not account for participants' underlying diseases or medication use, which may affect kidney function, diabetes, and hypertension. Second, lifestyle factors—such as smoking, alcohol consumption, and physical activity—were not evaluated. Third, since all participants were Korean, the results may not generalize to other populations. Lastly, due to limited access to InBody’s proprietary equations, it was unclear what caused discrepancies with DEXA. Future studies should increase sample diversity and ensure transparency in measurement algorithms.
- NOTES
- NOTES
-
AUTHOR CONTRIBUTIONS Dr. Byungwook YOO had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors reviewed this manuscript and agreed to individual contributions.
Conceptualization: SYL, HS, and BY. Data curation: SYL, SHB. Formal analysis: HS, JYS. Methodology: SYL, SHB, and BY. Supervision: SYL, BY. Writing–original draft: SYL, HS. Writing–review & editing: all authors.
CONFLICTS OF INTEREST No existing or potential conflict of interest relevant to this article was reported.
FUNDING None.
DATA AVAILABILITY The data presented in this study are available upon reasonable request from the corresponding author.
Fig. 1.
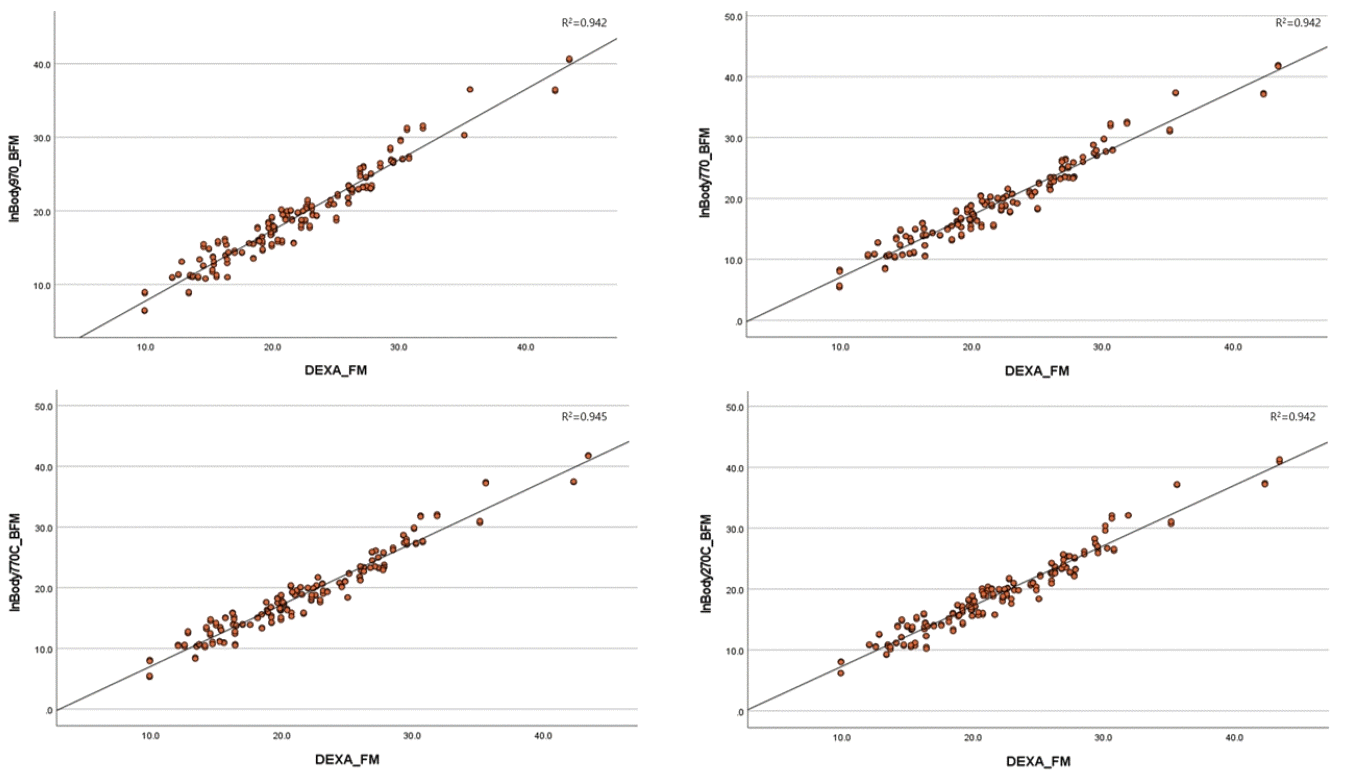
Fig. 2.
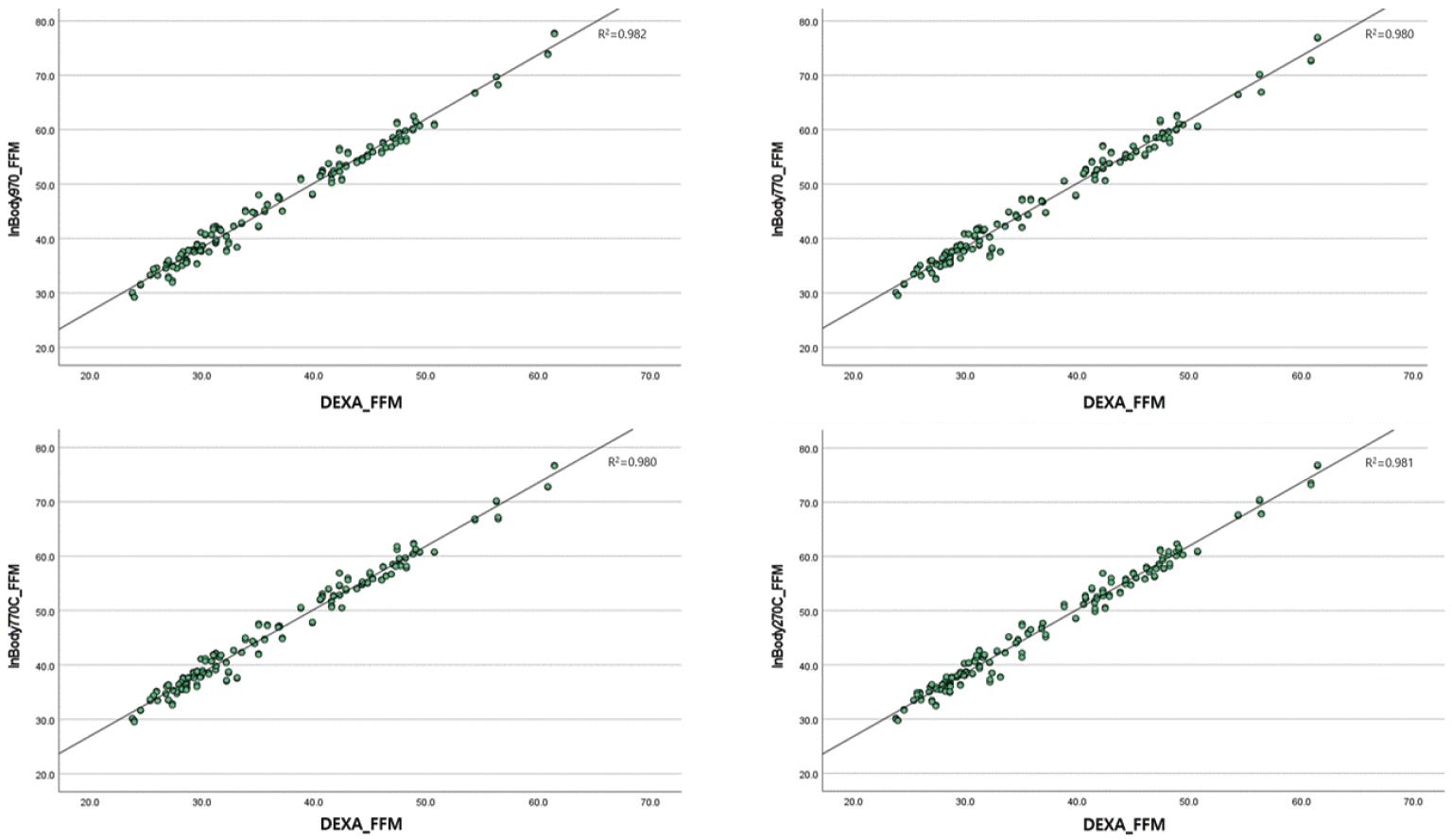
Fig. 3.
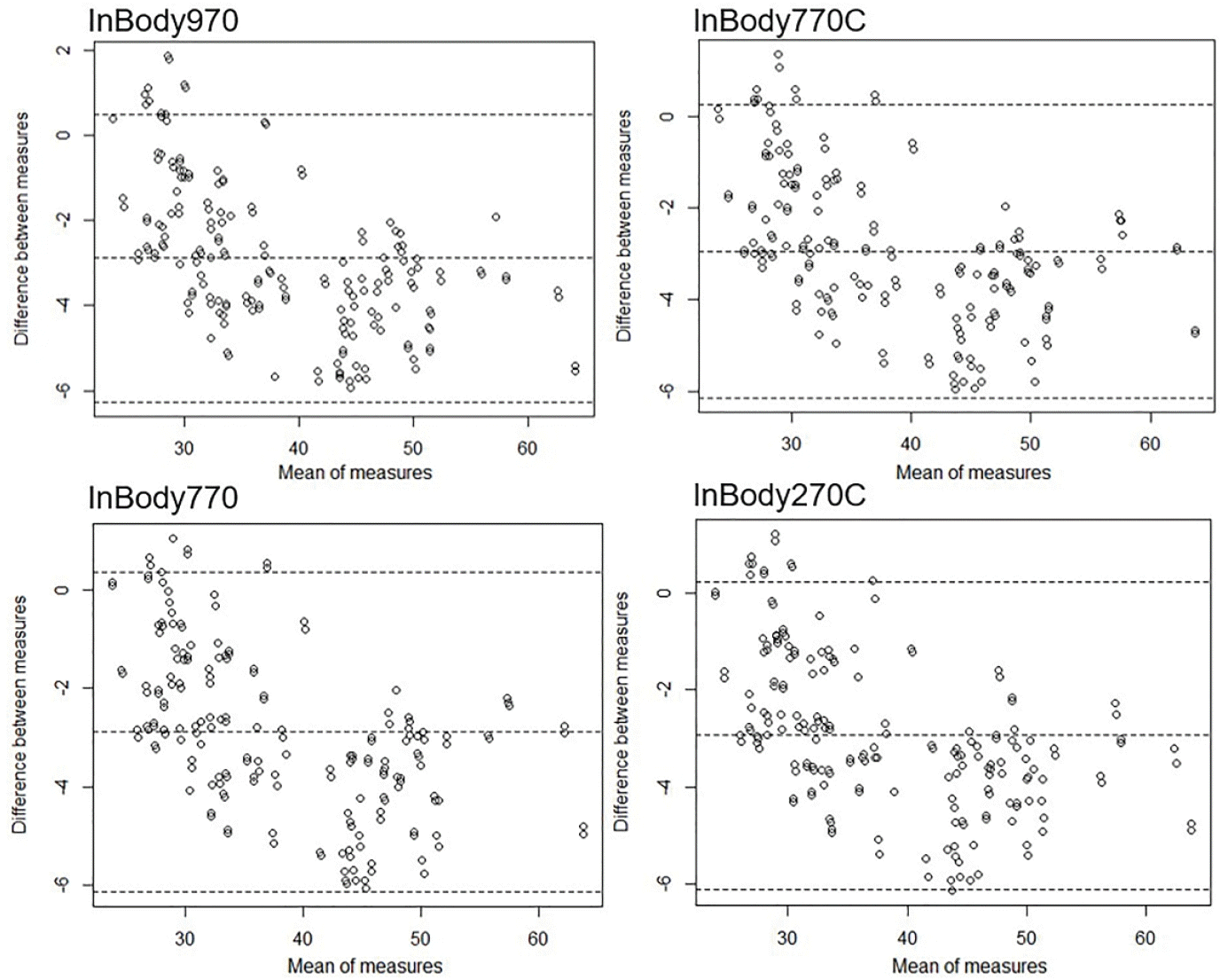
Table 1.
| Characteristic | Total (n=100) | Male (n=42) | Female (n=58) | P-valuea |
|---|---|---|---|---|
| Age (yr) | 41.3±11.3 | 39.0±10.8 | 43.0±11.3 | 0.01 |
| Weight (kg) | 65.8±13.6 | 76.4±11.5 | 58.1±9.2 | <0.05 |
| Height (cm) | 166.1±9.1 | 174.9±5.7 | 159.8±5.0 | <0.05 |
| BMI (kg/m2) | 23.7±3.5 | 24.9±3.0 | 22.8±3.6 | 0.0004 |
Table 2.
Table 3.
- REFERENCES
- REFERENCES
- 1. Lee EJ, Kim DK, Yoo SM, Kim KN, Lee SY. Association of visceral fat area measured by InBody 720 with the results measured by CT, DEXA and anthropometric measurement. Korean J Fam Med 2010;31(3):190-7.
[Article]2. Tauber RN, Camic CL, Zhang S, Chomentowski PJ 3rd. Comparison of multi-frequency bioelectrical impedance and dual-energy X-ray absorptiometry to assess body composition in college-aged adults. Int J Exerc Sci 2020;13(4):1595-604.
[Article] [PubMed] [PMC]3. Gonzalez MC, Heymsfield SB. Bioelectrical impedance analysis for diagnosing sarcopenia and cachexia: what are we really estimating? J Cachexia Sarcopenia Muscle 2017;8(2):187-9.
[Article] [PubMed] [PMC]4. Schiavo L, Scalera G, Pilone V, De Sena G, Iannelli A, Barbarisi A. Fat mass, fat-free mass, and resting metabolic rate in weight-stable sleeve gastrectomy patients compared with weight-stable nonoperated patients. Surg Obes Relat Dis 2017;13(10):1692-9.
[Article] [PubMed]5. Schiavo L, Pilone V, Tramontano S, Rossetti G, Iannelli A. May bioelectrical impedance analysis method be used in alternative to the dual-energy X-ray absorptiometry in the assessment of fat mass and fat-free mass in patients with obesity? Pros, cons, and perspectives. Obes Surg 2020;30(8):3212-5.
[Article] [PubMed]6. Gil JW, Park JH, Park MH, Park CY, Kim SY, Shin DW, et al. Estimated exposure dose and usage of radiological examination of the National Health Screening. J Radiat Prot 2014;39(3):142-9.
[Article]7. Kyle UG, Genton L, Hans D, Pichard C. Validation of a bioelectrical impedance analysis equation to predict appendicular skeletal muscle mass (ASMM). Clin Nutr 2003;22(6):537-43.
[Article] [PubMed]8. Sergi G, De Rui M, Veronese N, Bolzetta F, Berton L, Carraro S, et al. Assessing appendicular skeletal muscle mass with bioelectrical impedance analysis in free-living Caucasian older adults. Clin Nutr 2015;34(4):667-73.
[Article] [PubMed]9. Janssen I, Heymsfield SB, Baumgartner RN, Ross R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J Appl Physiol (1985) 2000;89(2):465-71.
[Article] [PubMed]11. Meier NF, Bai Y, Wang C, Lee DC. Validation of a multielectrode bioelectrical impedance analyzer with a dual-energy X-ray absorptiometer for the assessment of body composition in older adults. J Aging Phys Act 2020;28(4):598-604.
[Article] [PubMed]12. Morgan SL, Prater GL. Quality in dual-energy X-ray absorptiometry scans. Bone 2017;104:13-28.
[Article] [PubMed]13. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1(8476):307-10.
[Article] [PubMed]14. Lin LI. A concordance correlation coefficient to evaluate reproducibility. Biometrics 1989;45(1):255-68.
[Article] [PubMed]15. Vetter TR, Schober P. Agreement analysis: what he said, she said versus you said. Anesth Analg 2018;126(6):2123-8.
[Article] [PubMed]16. McBride GB. A proposal for strength-of-agreement criteria for Lin's Concordance Correlation Coefficient. National Institute of Water & Atmospheric Research Ltd.; Report No.: NIWA Client Report: HAM2005-062.17. Malbrain ML, Huygh J, Dabrowski W, De Waele JJ, Staelens A, Wauters J. The use of bio-electrical impedance analysis (BIA) to guide fluid management, resuscitation and deresuscitation in critically ill patients: a bench-to-bedside review. Anaesthesiol Intensive Ther 2014;46(5):381-91.
[Article] [PubMed]18. Park SS, Lim S, Kim H, Kim KM. Comparison of two DXA systems, Hologic Horizon W and GE Lunar Prodigy, for assessing body composition in healthy Korean adults. Endocrinol Metab (Seoul) 2021;36(6):1219-31.
[Article] [PubMed] [PMC]19. Tothill P, Hannan WJ, Wilkinson S. Comparisons between a pencil beam and two fan beam dual energy X-ray absorptiometers used for measuring total body bone and soft tissue. Br J Radiol 2001;74(878):166-76.
[Article] [PubMed]20. Watson LPE, Venables MC, Murgatroyd PR. An investigation into the differences in bone density and body composition measurements between 2 GE Lunar densitometers and their comparison to a 4-component model. J Clin Densitom 2017;20(4):498-506.
[Article] [PubMed]21. Kaminsky LA, Ozemek C, Williams KL, Byun W. Precision of total and regional body fat estimates from dual-energy X-ray absorptiometer measurements. J Nutr Health Aging 2014;18(6):591-4.
[Article] [PubMed]22. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39(4):412-23.
[Article] [PubMed] [PMC]23. Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc 2004;52(1):80-5.
[Article] [PubMed]24. Ha YC, Kim S, Yoo JI. Open, active-controlled clinical study to evaluate the correlation between whole body DEXA and BIA muscle measurements. J Bone Metab 2024;31(3):219-27.
[Article] [PubMed] [PMC]25. Fang WH, Yang JR, Lin CY, Hsiao PJ, Tu MY, Chen CF, et al. Accuracy augmentation of body composition measurement by bioelectrical impedance analyzer in elderly population. Medicine (Baltimore) 2020;99(7):e19103.
[Article] [PubMed] [PMC]26. Kim M, Shinkai S, Murayama H, Mori S. Comparison of segmental multifrequency bioelectrical impedance analysis with dual-energy X-ray absorptiometry for the assessment of body composition in a community-dwelling older population. Geriatr Gerontol Int 2015;15(8):1013-22.
[Article] [PubMed]27. Lee SY, Ahn S, Kim YJ, Ji MJ, Kim KM, Choi SH, et al. Comparison between dual-energy X-ray absorptiometry and bioelectrical impedance analyses for accuracy in measuring whole body muscle mass and appendicular skeletal muscle mass. Nutrients 2018;10(6):738.
[Article] [PubMed] [PMC]28. Anderson LJ, Erceg DN, Schroeder ET. Utility of multifrequency bioelectrical impedance compared with dual-energy x-ray absorptiometry for assessment of total and regional body composition varies between men and women. Nutr Res 2012;32(7):479-85.
[Article] [PubMed]29. Wang H, Hai S, Cao L, Zhou J, Liu P, Dong BR. Estimation of prevalence of sarcopenia by using a new bioelectrical impedance analysis in Chinese community-dwelling elderly people. BMC Geriatr 2016;16(1):216.
[Article] [PubMed] [PMC]30. Buckinx F, Reginster JY, Dardenne N, Croisiser JL, Kaux JF, Beaudart C, et al. Concordance between muscle mass assessed by bioelectrical impedance analysis and by dual energy X-ray absorptiometry: a cross-sectional study. BMC Musculoskelet Disord 2015;16:60.
[Article] [PubMed] [PMC]31. Yi Y, Baek JY, Lee E, Jung HW, Jang IY. A comparative study of high-frequency bioelectrical impedance analysis and dual-energy X-ray absorptiometry for estimating body composition. Life (Basel) 2022;12(7):994.
[Article] [PubMed] [PMC]32. Santilli V, Bernetti A, Mangone M, Paoloni M. Clinical definition of sarcopenia. Clin Cases Miner Bone Metab 2014;11(3):177-80.
[Article] [PubMed] [PMC]33. Anker SD, Morley JE, von Haehling S. Welcome to the ICD-10 code for sarcopenia. J Cachexia Sarcopenia Muscle 2016;7(5):512-4.
[Article] [PubMed] [PMC]
