Antimicrobial Resistance in Human Health, Crisis, and Seizing Opportunities: A Comprehensive Literature Review
Article information
Abstract
Antimicrobial resistance (AMR) poses a significant threat to global health, with the potential to reverse decades of medical progress. AMR occurs when microorganisms evolve to resist the effects of antimicrobial drugs, rendering treatments ineffective. This phenomenon is driven by mechanisms such as genetic mutations, horizontal gene transfer, and the overuse of antimicrobials in both human medicine and agriculture. The economic impact of AMR is profound, with projections indicating a potential reduction in global gross domestic product by 2% to 3.5% by 2050, disproportionately affecting low- and middle-income countries (LMICs) due to weaker healthcare infrastructure and higher rates of infectious diseases. Addressing AMR requires a multifaceted approach, including improved antimicrobial stewardship, enhanced surveillance, and the development of new treatments. Surveillance systems, particularly in LMICs, are often inadequate, highlighting the need for global collaboration and data-sharing networks like the Latin American Antimicrobial Resistance Surveillance Network (ReLAVRA). Additionally, the interconnected use of antimicrobials in humans, animals, and the environment necessitates a One Health approach to effectively combat AMR. Preventive measures such as vaccination, infection control practices, and rapid diagnostic tests are crucial in reducing the spread of resistant infections. Antimicrobial stewardship programs and precision medicine offer promising strategies to minimize unnecessary antimicrobial use and slow resistance development. However, the development of new antimicrobials remains a critical challenge, requiring innovative models of collaboration between academia, industry, and governments.
ANTIMICROBIAL RESISTANCE: AN ESCALATING GLOBAL CRISIS
Antimicrobials have been a cornerstone of modern medicine, drastically reducing the global burden of infectious diseases and saving millions of lives [1]. However, the rise of antimicrobial resistance (AMR) now threatens to undo these healthcare advancements. AMR occurs when microorganisms like bacteria, viruses, fungi, and parasites evolve to resist the drugs designed to kill them, rendering treatments ineffective [1]. This phenomenon has become a global crisis, affecting countries of all income levels, In 2014, an estimated 700,000 deaths worldwide were linked to infections caused by antibiotic-resistant organisms [2], and this number is projected to rise to 10 million annually by 2050 if no action is taken [3]. The implications of AMR extend beyond public health, with far-reaching consequences for global economies and healthcare systems.
The economic impact of AMR is staggering, with predictions suggesting it could reduce global gross domestic product (GDP) by 2% to 3.5% by 2050 [4], equivalent to losses of 60 to 100 trillion US dollars. Low- and middle-income countries (LMICs) are expected to bear the brunt of this burden due to weaker healthcare infrastructure, higher rates of infectious diseases, and limited access to effective treatments. In these regions, the lack of robust data and research further obscures the true scale of the problem. As resistance to second- and third-generation antibiotics grows, the situation is likely to worsen. Critically ill patients may no longer respond to available treatments, forcing healthcare providers to rely on supportive care alone. This shift would lead to longer hospital stays, increased healthcare costs, and higher mortality rates, placing immense strain on already fragile systems.
Addressing AMR requires urgent, coordinated global action. The development of new antimicrobials has stagnated due to high costs and low financial incentives for pharmaceutical companies, leaving a critical gap in the pipeline for innovative treatments. To combat this crisis, efforts must focus on improving antibiotic stewardship, strengthening healthcare infrastructure, and investing in research and development. International collaboration is essential to promote responsible antibiotic use, enhance surveillance, and support LMICs in building capacity to manage AMR, without immediate and sustained intervention, the world risks entering a post-antibiotic era where common infections become untreatable, reversing decades of medical progress and imposing an insurmountable burden on global health and economies.
MECHANISMS OF ANTIMICROBIAL RESISTANCE
AMR arises primarily due to the selective pressure exerted on microorganisms when they are exposed to antimicrobial agents. Bacteria develop resistance through various mechanisms [5], including the production of enzymes that inactivate drugs, alterations to target sites that prevent antimicrobial binding, changes in metabolic pathways that bypass drug effects, reduced outer membrane permeability that limits drug entry, and the activation of efflux pumps that expel antimicrobials from the cell, these mechanisms often work in combination, enabling bacteria to resist multiple classes of antimicrobials simultaneously. Genetic variation, driven by mutations, DNA rearrangements, and horizontal gene transfer, plays a central role in the evolution of resistance, allowing bacteria to adapt and survive in the presence of drugs.
Horizontal gene transfer is a particularly significant factor in the spread of AMR. Bacteria can acquire resistance genes from other organisms through mobile genetic elements like plasmids, transposons, and integrons [6]. These elements can carry multiple resistance genes, enabling rapid dissemination of resistance traits across bacterial populations and even between species. Point mutations and larger genetic changes also contribute to resistance by altering the structure or function of proteins targeted by antimicrobials. Even a single mutation can provide a survival advantage, allowing resistant bacteria to thrive while susceptible organisms are eliminated.
The widespread use of antimicrobials creates environments where resistant bacteria have a selective advantage, leading to the dominance of resistant strains. This underscores the importance of using antimicrobials judiciously to slow the development and spread of AMR [7]. Understanding the diverse mechanisms of resistance is critical for developing effective strategies to combat AMR and preserve the efficacy of antimicrobials for future generations. Addressing this global challenge requires a multifaceted approach, including improved stewardship of existing drugs, the development of new therapies, and enhanced surveillance of resistant pathogens.
ANTIMICROBIAL RESISTANCE IN HUMANS AND ANIMALS
The use of antimicrobials in human medicine has significantly reduced the burden of infectious diseases and enabled advanced medical procedures like organ transplants and surgeries [8]. However, their overuse, misuse, and improper administration in clinical settings have become major contributors to the rise of AMR in human populations. AMR occurs when pathogens evolve to resist the effects of medications, making infections harder to treat and posing a serious threat to global public health. Similarly, antimicrobials are widely used in animal agriculture, particularly in livestock farming, where they are often administered at sub-therapeutic doses to promote growth a practice still common in the Americas and Asia, though banned in the European Union since 2006 [9].
In modern livestock systems, animals are often kept in close confinement, leading to high levels of endemic disease. Treating individual animals is frequently uneconomical, so entire groups are given blanket antimicrobial treatments through feed or water. This overuse accelerates the development of resistant bacteria, which can spread to humans through direct contact, contaminated food, or environmental pathways like agricultural runoff. The interconnected use of antimicrobials in humans, animals, and the environment creates significant selective pressure for AMR, The One Health approach is a collaborative, multisectoral framework that recognizes the interconnectedness of human, animal, and environmental health. It emphasizes coordinated interventions to address shared threats, such as AMR, zoonotic diseases, and ecosystem degradation [10].
To combat AMR, integrated surveillance systems are essential to monitor resistance patterns in hospitals, communities, and food-producing animals. National and global data networks could help identify emerging resistance trends and index cases, enabling timely interventions. Addressing AMR also requires stricter regulations on antimicrobial use, reduced reliance on these drugs in agriculture, and increased public awareness by taking a coordinated, multidisciplinary approach, we can mitigate the spread of AMR, preserve the effectiveness of antimicrobials, and safeguard global health for future generations.
SPREAD OF ANTIMICROBIAL RESISTANCE
The transmission of AMR between humans and animals is a complex issue influenced by the interplay of human, animal, and environmental factors. Studies have shown a correlation between antimicrobial use in animals and the rise of resistant bacteria in humans, though the relationships are not always consistent. For example, while some research indicates that livestock and humans may not share the same bacterial strains, the emergence of livestock-associated methicillin-resistant Staphylococcus aureus (MRSA) [11], such as clonal complex 398, demonstrates that resistant bacteria can spread across species. However, aggregated data often reveal inconsistencies in resistance patterns, highlighting the need for more detailed, context-specific studies to better understand these dynamics. Observational research is further limited by confounding factors, such as differences in antimicrobial use practices and environmental conditions, underscoring the importance of cautious interpretation and targeted investigations.
ANTIMICROBIAL RESISTANCE TRANSMISSION THROUGH ANIMAL-DERIVED FOOD (Fig. 1)
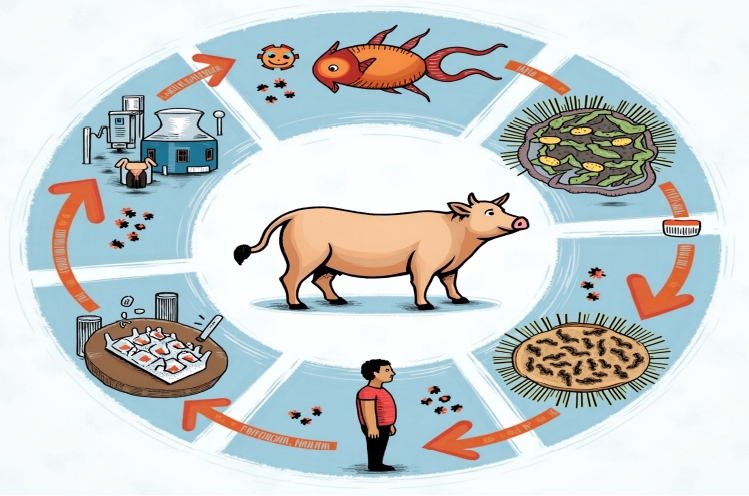
Antimicrobial resistance (AMR) in food via animals. The figure illustrates the transmission pathways of AMR from animals to humans through food consumption, highlighting the interconnectedness of human, animal, and environmental health in the spread of AMR.
Advances in genetic and epidemiological tools, including whole-genome sequencing, have provided insights into the origins and transmission of resistant pathogens [12]. For instance, some MRSA lineages in humans may have originated from animals, while others suggest human-to-animal transmission. Similarly, while certain resistant Escherichia coli strains can spread from livestock to humans via food, resistance genes are often carried on distinct plasmids in each population, limiting direct transmission. These findings highlight the complexity of AMR transmission, which varies by pathogen, resistance genes, and ecological context. Addressing this challenge requires a One Health approach, integrating human, animal, and environmental health, as well as interdisciplinary collaboration to develop effective strategies for controlling AMR.
ANTIMICROBIAL RESISTANCE SURVEILLANCE: A CRITICAL STEP IN COMBATING ANTIMICROBIAL RESISTANCE
Addressing AMR begins with understanding its scale, distribution, and causes, which is critical for designing effective public health policies. In the regions where surveillance is often limited to smaller, hospital-based studies, which may overestimate AMR prevalence due to their focus on healthcare-associated infections, to address this, there is a need for national and regional collaborative surveillance networks that facilitate data sharing and provide a more accurate picture of AMR trends. Latin American Network for Antimicrobial Resistance Surveillance (ReLAVRA) aggregates data from National Reference Laboratories across Latin America, significantly improving the region’s ability to detect and monitor AMR [13]. This initiative has guided policies on antimicrobial use and demonstrated the value of regional networks in strengthening surveillance. Expanding similar efforts to regions like Africa and South-East Asia is essential for building a global response to AMR [14]. Establishing these networks would improve monitoring, support data-driven policymaking, and promote responsible antimicrobial use.
To ensure the success of these networks, challenges such as limited laboratory capacity, inadequate funding, and a lack of trained personnel must be addressed. Investing in infrastructure, building technical expertise, and leveraging digital technologies can enhance surveillance efforts. Engaging policymakers with accurate data is also crucial for developing evidence-based interventions, such as guidelines for antimicrobial use and infection prevention measures, fostering collaboration and sharing surveillance findings globally [15], we can better understand AMR trends and develop coordinated strategies to combat resistance. Strengthening AMR surveillance worldwide is vital to safeguarding the effectiveness of antimicrobials for future generations.
THE IMPORTANCE OF TECHNOLOGICAL INTEGRATION IN EPIDEMIOLOGICAL SURVEILLANCE
Table 1 [16-25] provides an overview of key global and national initiatives, stakeholders, aims, information requirements, and challenges related to addressing AMR. It highlights the multi-sectoral nature of AMR efforts, involving human health, animal health, agriculture, and environmental sectors, as well as the importance of a One Health approach. Table 1 also identifies gaps and challenges, such as inconsistent data collection, limited resources, regulatory hurdles, and the need for sustained funding and innovation. Sources include reports from global organizations (e.g., World Health Organization, Food and Agriculture Organization, World Organization for Animal Health), national agencies (e.g., Centers for Disease Control and Prevention, National Institutes of Health) [26], and historical context, emphasizing the ongoing and historical efforts to combat AMR.
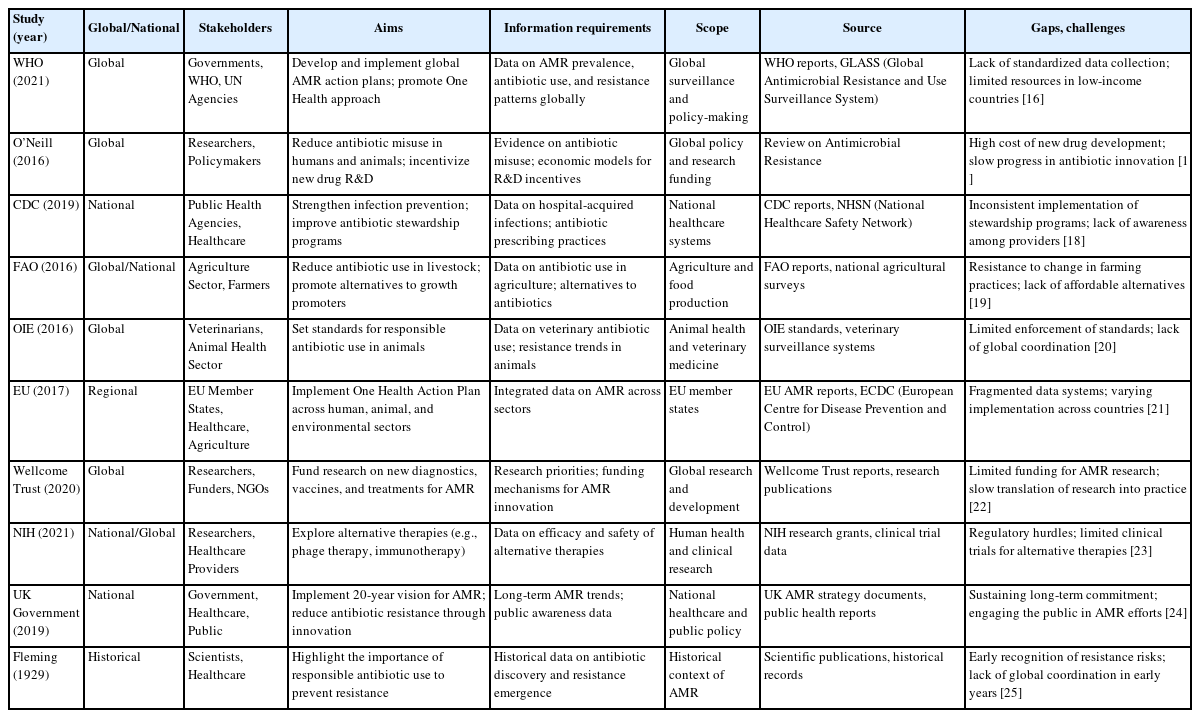
Comprehensive framing of the approach to the AMR problem according to stakeholder groups, including their common aims, gaps, and challenges
Understanding the global burden, distribution, and drivers of AMR remains a significant challenge, despite increasing attention to the issue. Current evidence on the link between antimicrobial use and resistance, such as MRSA, is often limited by poor reporting in individual studies. This underscores the urgent need for high-quality epidemiological data, particularly for high-risk populations and globally representative samples. Population-level studies are critical for assessing AMR prevalence, guiding context-specific treatment strategies, and informing interventions to combat resistant pathogens. Strengthening existing surveillance systems is essential to achieving these goals.
With few new treatments for resistant infections, understanding the factors driving AMR is more important than ever. While reducing antimicrobial misuse and overuse is a key focus, the lack of evidence-based guidance on effective interventions in hospital and community settings hinders progress. Large-scale epidemiological studies are needed to explore the relationship between AMR and patterns of antimicrobial use, informing stewardship programs for both human and agricultural practices. Such research could also identify high-risk populations and transmission pathways, enabling targeted prevention and treatment strategies.
Integrated approaches that combine human, animal, and environmental data using standardized methods can help trace the evolutionary origins of resistant pathogens and identify transmission routes [27]. Additionally, electronic health records (EHRs) offer valuable insights into prescribing practices and resistance trends, though their utility is limited by disparities in reporting and infrastructure, particularly in LMICs. Expanding surveillance to include animal infections and veterinary practices, alongside centralized data sharing, could provide a more comprehensive understanding of AMR on a national or regional scale. Prospective, longitudinal studies are also critical for real-time monitoring of resistance development and the factors influencing its spread, by addressing these gaps, we can better predict, prevent, and respond to the growing threat of AMR.
PREVENTIVE MEASUREMENTS AND MANAGEMENT OF ANTIMICROBIAL RESISTANCE
Developing new antibiotics is crucial but risks fueling an evolutionary “arms race.” Each new antibiotic puts pressure on microbes, favoring resistant strains [7]. This creates a cycle where microbes adapt faster than drugs can evolve. Alternative strategies aim to break this cycle. For example, targeting virulence factors (not survival) may reduce resistance pressure. Phage therapy uses viruses that evolve with bacteria, offering adaptable solutions [28]. Combination therapies or resistance-blocking adjuvants (like efflux pump inhibitors) can also prolong antibiotic effectiveness [29]. These approaches don’t stop resistance entirely but shift from aggressive “kill strategies” to sustainable management. More research on evolution and host-pathogen interactions is needed to improve these methods and slow resistance.
Efforts to combat AMR must focus not only on understanding its causes but also on implementing effective prevention and treatment strategies. A key approach is reducing the spread of infections, which would decrease the need for antimicrobial use and, in turn, slow the development of resistance. This can be achieved through measures such as vaccination, safe food handling, infection control practices (e.g., hand hygiene, barrier precautions, and isolating infected patients) [30], and improved waste management. These strategies are vital in hospitals, communities, agricultural settings, and the environment. However, challenges persist, particularly in LMICs, where limited resources, weak health infrastructure, and human resource constraints hinder the implementation of basic interventions like hygiene practices and antimicrobial stewardship. Tailoring AMR prevention efforts to local contexts is essential to ensure they are both feasible and effective.
PREVENTION AND MANAGEMENT OF ANTIMICROBIAL RESISTANCE (Fig. 2)
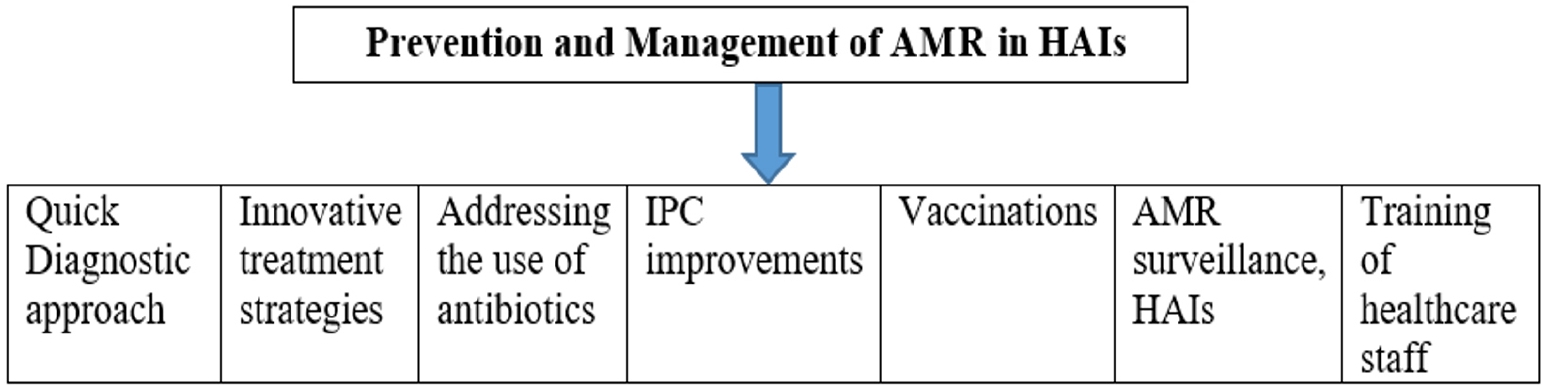
The figure outlines key strategies for preventing and managing AMR, including vaccination, infection control practices, rapid diagnostics, and antimicrobial stewardship programs, emphasizing the need for a coordinated, multisectoral approach to combat AMR. AMR, antimicrobial resistance, HAIs, healthcare-associated infections; IPC, infection prevention and control.
A critical tool in the fight against AMR is the development of rapid diagnostic tests that can quickly identify bacterial infections and resistance markers. Such tests would be invaluable in both high- and low-resource settings. Currently, the lack of reliable point-of-care diagnostics often leads to the empirical use of antimicrobials while awaiting microbiological test results. This practice, combined with suboptimal dosing or treatment durations, can promote the emergence of resistant strains. Public behavior also plays a role, patients often stop treatment prematurely, which encourages the survival of resistant organisms. Public education campaigns on the proper use of antimicrobials are crucial, yet only half of countries have implemented such initiatives, with most being high-income nations. Additionally, the widespread availability of substandard or counterfeit antimicrobials exacerbates the problem, undermining stewardship efforts and endangering lives.
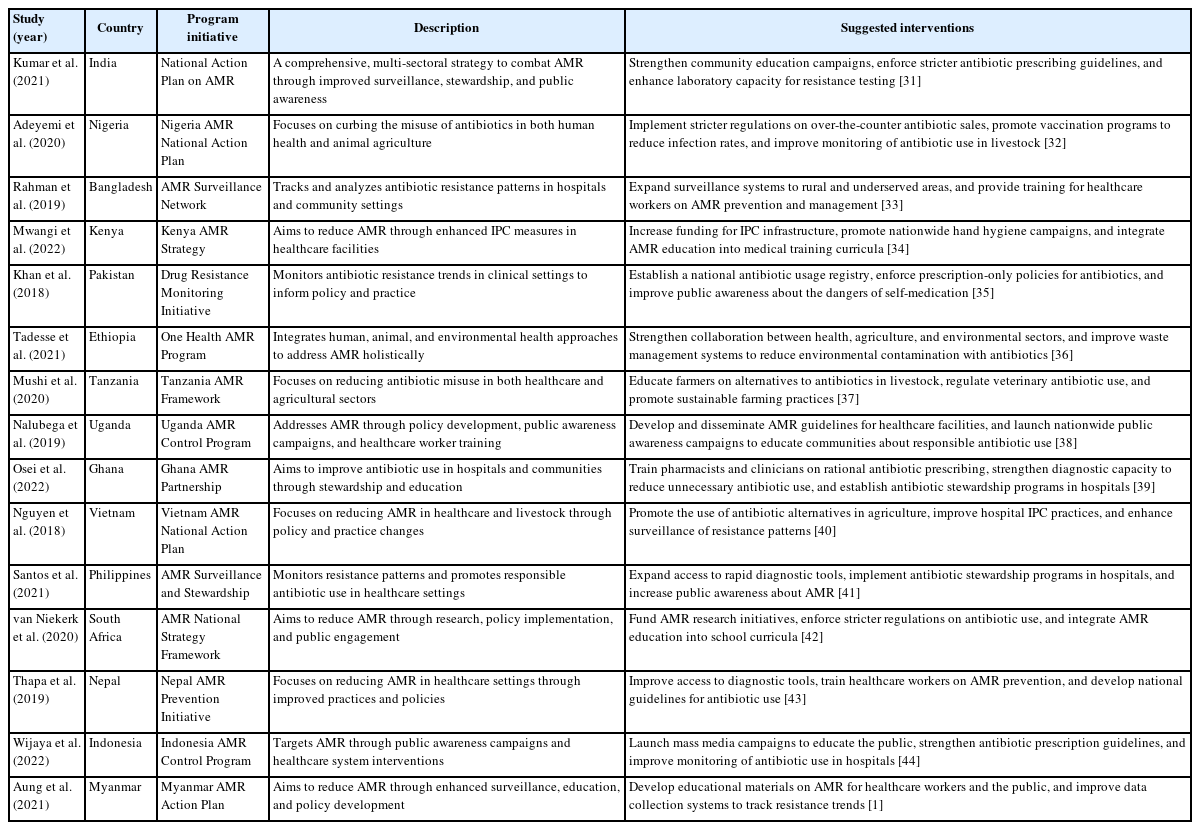
Table 2 [1,31-44] summarizes key studies, national programs, and suggested interventions to address AMR across various LMICs. Each initiative reflects a multi-sectoral approach, emphasizing surveillance, stewardship, public awareness, and policy enforcement to combat AMR. The suggested interventions highlight the need for strengthened healthcare systems, improved regulatory frameworks, and community engagement to mitigate the growing threat of AMR globally. Data sources include peer-reviewed studies and national action plans published between 2018 and 2022.
Globalization further complicates AMR control by facilitating the spread of resistant infections across borders [45], population movement can introduce drug-resistant pathogens from high-prevalence areas to regions with lower resistance rates [46]. However, quantifying this risk remains difficult, highlighting the need for enhanced international surveillance. Advanced technologies, such as next-generation sequencing, can help trace the origins of resistant organisms and assess the role of population movement in their spread. In LMICs, rapid urbanization and overcrowding, coupled with poor sanitation, create environments conducive to the transmission of resistant infections. Addressing these challenges requires a coordinated, multisectoral approach involving stakeholders worldwide.
Antimicrobial stewardship programs (ASPs) are a cornerstone of AMR prevention [47]. These programs promote the judicious use of antimicrobials through evidence-based treatment guidelines. EHR systems have proven effective in supporting ASPs by enabling clinicians to specify indications for antibiotic use, review microbiology results, and adjust or discontinue treatment as needed. EHRs can also guide prescribing practices by recommending preferred regimens based on local resistance patterns. However, the success of ASPs depends on comprehensive ecological and epidemiological data, which are often lacking. Financial barriers, particularly in LMICs, also limit their implementation, despite evidence that ASPs are cost-effective in the long term. To maximize their impact, ASPs should be integrated with other initiatives, such as hand hygiene campaigns, pharmacy management, and laboratory quality control.
Despite these challenges, several LMICs have successfully implemented ASPs by adopting context-specific strategies. A notable example is Nigeria’s National Action Plan on AMR (2020), which achieved a 35% reduction in unnecessary antibiotic use within 2 years [48] through.
1- Diagnostic capacity strengthening: Introduction of affordable rapid tests to guide prescribing
2- Prescriber engagement: Monthly audit-and-feedback sessions with peer comparison
3- Community involvement: Public awareness campaigns to discourage self-medication
This demonstrates that tailored, low-cost interventions even in resource-limited settings can significantly improve antibiotic stewardship.
Precision medicine offers another promising avenue for reducing AMR. By enabling targeted therapies based on the specific infective organism and the patient’s susceptibility, precision medicine can minimize unnecessary antimicrobial use and slow resistance development. However, this approach relies on the availability of accurate, rapid, and affordable diagnostic tests, which remain a challenge in many settings.
Finally, the development of new antimicrobials is urgently needed. Since 1987, no new class of antibiotics has been discovered, and drug development has largely focused on modifying existing compounds [49]. The high costs and low success rates of antibiotic development have discouraged pharmaceutical companies from investing in this area, leaving academic institutions unable to advance new drugs without industry support. A new model for drug development is essential—one that involves collaboration between academia, pharmaceutical companies, and governments to share risks and costs. This approach, recommended by the O’Neill Review on AMR, could incentivize the creation of new treatments for resistant and multidrug-resistant infections [50], provided their use is tightly controlled to preserve their effectiveness. Tackling AMR requires a multifaceted strategy that combines prevention, stewardship, innovation, and global cooperation, addressing the root causes of resistance and investing in new tools and treatments, we can mitigate the growing threat of AMR and safeguard public health for future generations.
CONCLUSION
AMR is a critical global health threat that demands urgent, coordinated action to prevent devastating economic and social impacts. Addressing AMR requires improved stewardship, enhanced surveillance, and the development of new treatments, supported by a One Health approach. Preventive measures like vaccination, infection control, and rapid diagnostics are essential to curb resistance. Collaboration between academia, industry, and governments is vital to innovate and preserve effective treatments. Without immediate intervention, the world risks a post-antibiotic era where common infections become untreatable, reversing decades of medical progress.
Notes
AUTHOR CONTRIBUTIONS
Dr. Muhammad Ajmal DINA had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors reviewed this manuscript and agreed to individual contributions.
Conceptualization: MAD. Data curation: MAD. Formal analysis: AA. Methodology: AA. Writing–original draft: AA. Writing–review & editing: all authors.
CONFLICTS OF INTEREST
No existing or potential conflict of interest relevant to this article was reported.
FUNDING
None.
DATA AVAILABILITY
No datasets used and/or analyzed during the current study.
ACKNOWLEDGMENTS
We would like to acknowledge the colleagues of my department for their support.