Clinical Validation of a Mobile-Based ACR (Urine Albumin-to-Creatinine Ratio) Test Kit: Comparative Analysis with Benchtop Devices
Article information
Abstract
Background
Albuminuria, a marker for diabetes complications and kidney disease, is typically tested in hospitals, limiting patient monitoring. The mobile-based ACR (urine albumin-to-creatinine ratio) test, QSCheck-UISACR, was developed for easy detection of albuminuria. This study aimed to validate its clinical efficacy compared to a conventional benchtop device (Cybow R-50S) for early kidney disease diagnosis.
Methods
Conducted in three hospitals with 303 kidney disease patients, urine samples were tested using both the mobile ACR kit and the benchtop device. Parameters measured included microalbumin, creatinine, and their ratio (albumin-to-creatinine ratio). Exact Agreement and Within One Block Agreement were calculated to compare methods.
Results
Data collected from three university hospitals were aggregated and analyzed, showing that the microalbumin Exact Agreement was 73.3%, with a Within One Block Agreement of 96.0%. For creatinine, the Exact Agreement was 75.6%, and the Within One Block Agreement was 95.7%. Based on these two test results, the albumin-to-creatinine ratio analysis demonstrated an Exact Agreement of 78.5% and a Within One Block Agreement of 98.0%. These findings highlight the robust performance of the tests across the evaluated biomarkers.
Conclusions
The mobile-based ACR test kit demonstrated high accuracy and comparability to the benchtop device, suggesting its potential as a reliable tool for early kidney disease screening in primary care settings, enhancing patient accessibility and reducing healthcare costs.
INTRODUCTION
Diabetes is a significant chronic disease that can lead to complications such as kidney disease, neuropathy, and retinopathy, making early diagnosis and proper management crucial [1-3]. In South Korea, the rising prevalence of diabetes underscores the importance of primary care in early diagnosis and management, as primary care providers play a vital role in enhancing patient quality of life and preventing complications through timely intervention [4,5]. Particularly critical is the early diagnosis and management of kidney disease, as kidney function damage is irreversible and often necessitates costly treatments like dialysis or kidney transplantation in end-stage renal disease [6,7]. The Korean Society of Nephrology (KSN) reported in 2019 that the number of end-stage renal disease patients in Korea is increasing, imposing a substantial burden on health insurance [7,8]. Early detection and management can prevent disease progression, improve patient quality of life, and reduce both the economic burden on patients and the need for high-cost treatments [9,10].
The albumin-to-creatinine ratio (ACR) test is recommended for early screening of kidney disease, regardless of whether they have diabetes or not. This test measures the ACR in urine, providing a simple, non-invasive method to detect early kidney damage [11,12]. The guidelines of the KSN recommend regular ACR testing for high-risk groups, including diabetes and hypertension patients [13,14]. This test helps detect early changes in kidney disease, allowing for timely treatment and significantly improving patient outcomes [13,15].
However, precise quantitative ACR tests are difficult to use in primary care due to high costs and the need for outsourced testing. Traditional benchtop ACR tests are costly and time-consuming, with results taking days to weeks. This delay hinders early diagnosis and prompt treatment. Therefore, there is a growing need for a more affordable and rapid method, such as a digital camera-based ACR test kit [9,11]. While these products already exist, studies have shown their effectiveness as a screening tool compared to traditional quantitative analyzers, particularly in detecting albuminuria [16]. Recent advancements in mobile health (mHealth) technology have further increased accessibility, making it easier for both patients and healthcare providers to use these tools effectively [17,18]. Building on these findings, this study specifically focuses on evaluating their applicability and effectiveness in primary care settings, providing additional data on their clinical implementation in real-world clinical environments [9,11,13].
In response to this need, we conducted a study to compare the clinical efficacy of the digital camera-based ACR test kit with the traditional benchtop equipment used in hospitals. Specifically, we compared the clinical performance of the mobile digital camera-based QSTAG QSCheck-UISACR ACR test kit with traditional benchtop devices. The study aimed to evaluate the accuracy and reliability of the two digital camera-based ACR test kits compared to conventional benchtop ACR test equipment. By applying both testing methods in various clinical settings and comparing the results, we sought to demonstrate the efficiency of the digital camera-based kits in the early detection of kidney damage through measurements of albumin and creatinine levels and ratios.
METHODS
Study population
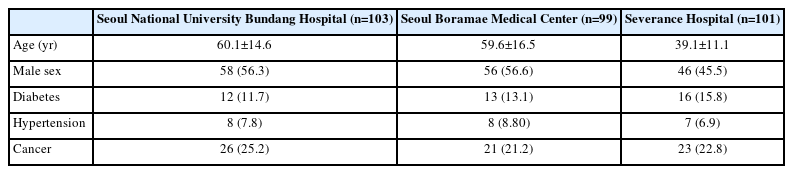
This study was conducted at three major hospitals in Seoul, targeting a total of 303 patients with kidney disease. The patients with kidney disease were as followed; undergoing monitoring for proteinuria, having a history of acute kidney injury, being diagnosed with chronic kidney disease and glomerulonephritis, or receiving a kidney transplantation. The study population included adults aged 19 years or older who were being followed up at the nephrology outpatient clinics of Seoul National University Bundang Hospital, Seoul Boramae Medical Center, and Severance Hospital. Patients considered eligible for the study were approached directly by medical staff in the outpatient clinic, where the research was explained to them and their consent was obtained before proceeding with the study. A total of 100 patients were recruited from each hospital based on the presence of proteinuria, excluding those undergoing dialysis or using diapers. The study emphasizes the importance of early diagnosis and management of kidney disease, aiming to evaluate the accuracy of a mobile-based ACR test kit compared to traditional benchtop equipment.
The inclusion criteria for the study were:
1. Adults aged 19 years or older.
2. Patients with kidney disease who were being followed up at the nephrology outpatient clinics of Seoul National University Bundang Hospital, Seoul Boramae Medical Center, and Severance Hospital.
3. Patients showing proteinuria, defined as a trace or higher result on a urine dipstick test.
The exclusion criteria were:
1. Patients undergoing dialysis.
2. Patients using diapers.
The study aimed to enroll a total of 100 subjects per clinical site, with a minimum of five subjects per concentration range. The basis for determining the number of subjects followed the CLSI EP12-A2 guidelines, selecting a minimum of 100 subjects per clinical site. The number of subjects per biomarker could overlap based on their values.
The target numbers for each biomarker concentration range per clinical site were as follows:
• For albumin concentrations of 1 mg/dL, 3 mg/dL, 8 mg/dL, and 15 mg/dL, each concentration range included more than 5 subjects per site, totaling a minimum of 100 subjects per site.
• For creatinine concentrations of 10 mg/dL, 50 mg/dL, 100 mg/dL, 200 mg/dL, and 300 mg/dL, each concentration range included more than five subjects per site, totaling a minimum of 100 subjects per site.
Ethics
The study protocol complied with the Declaration of Helsinki and received ethics approval from the institutional review board (IRB) of Seoul National University Bundang Hospital (IRB number: E-2209-780-352) which is integrated with Boramae medical center (IRB number: 20-2022-107) and Yonsei University Severance Hospital (IRB number: 1-2022-0051). Written informed consent was obtained from all patients.
Digital camera-based ACR test kit
The digital camera-based ACR test kit significantly enhances accuracy and usability by leveraging traditional urine chemistry test strips. Traditionally, these test strips required visual inspection and interpretation of color changes, which were subjective and prone to error. This innovative test kit employs a digital camera to capture high-resolution images, which are then processed by specialized software. The software accurately identifies and quantifies the color changes on the test strips, measuring the concentrations of albumin and creatinine. Based on these measurements, the software automatically calculates the ACR, eliminating the need for manual calculations and reducing the risk of human error. This automated process ensures consistent and objective analysis, providing more reliable results than traditional visual inspections. Consequently, the digital camera-based ACR test kit offers a more stable and accurate method for detecting and managing kidney disease, significantly improving early diagnosis and patient care in primary care settings.
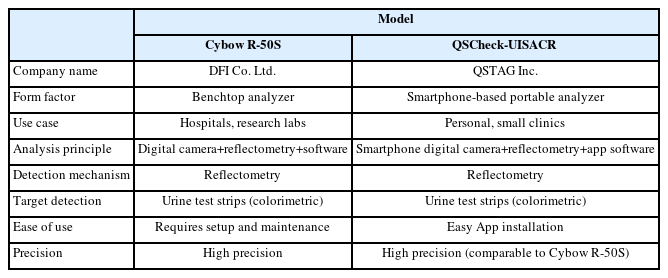
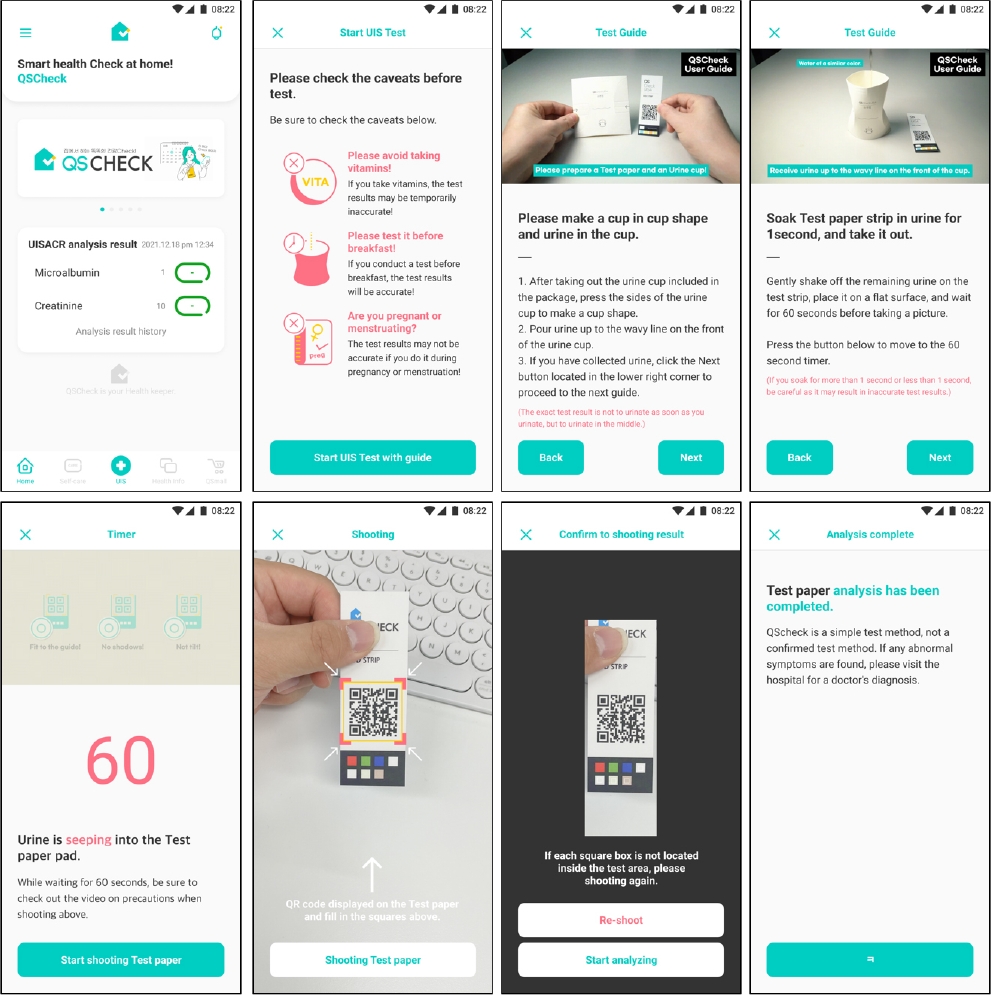
The comparison in Table 1 highlights the key differences and similarities between the Cybow R-50S and the QSTAG-UISACR. Both systems utilize reflectometry for detection and analyze colorimetric urine test strips. While the Cybow R-50S is a benchtop analyzer commonly used in hospitals and research labs, the QSTAG-UISACR is a smartphone-based portable analyzer suited for personal use and small clinics. Notably, QSTAG-UISACR utilizes high-resolution smartphone cameras and advanced software to provide quick results. Additionally, the QSTAG-UISACR is easier to use, requiring only simple app installation without the need for complex setup and maintenance as shown in Fig. 1.
Variables & outcomes
This study conducted evaluations to compare the two devices, focusing on the performance of measuring microalbumin and creatinine concentrations in urine, and based on these, assessing the equivalence of the ACR ratio. These evaluations aimed to compare the concordance and accuracy between the two testing methods. Statistical analysis was performed to evaluate this concordance and accuracy. Each test result was meticulously recorded, with concordance assessed using Exact Agreement (EA) and Within One Block Agreement (WOBA). EA refers to cases where the results were identical, while WOBA refers to results that fell within the same concentration range. All data were analyzed using SPSS software, with separate analyses conducted for each hospital and combined analyses for the overall dataset. Statistical significance was evaluated using the chi-square test and Pearson correlation coefficient, with a P-value of less than 0.05 considered statistically significant. The results of this study aim to validate the efficacy of the digital camera-based ACR test kit and assess its feasibility for use in primary care settings.
Evaluation
This study compared the clinical efficacy of a mobile-based ACR test kit (QSCheck-UISACR) with a benchtop device (Cybow R-50S) used in hospitals. The study was conducted on 303 patients with kidney disease at Seoul National University Bundang Hospital, Seoul Boramae Medical Center, and Severance Hospital. The study included data on average age, male proportion, and average urine albumin concentration as shown in Table 2. Urine samples from each patient were tested using two different methods.
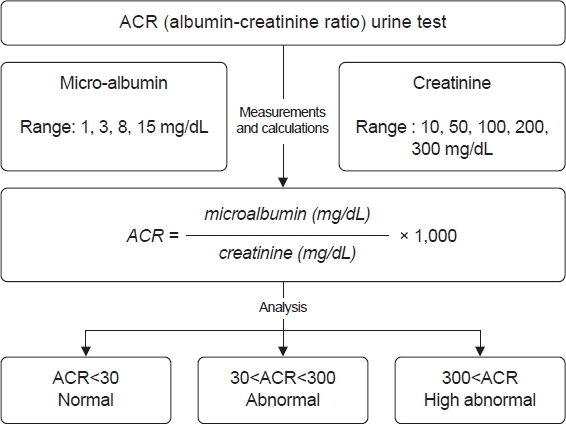
Fig. 2 shows the process and rationale behind the ACR urine test. This test measures the concentrations of microalbumin and creatinine in urine and calculates the ACR to assess kidney function and detect abnormalities.
Microalbumin concentration is measured within the range of 1, 3, 8, and 15 mg/dL. This range helps in detecting early stages of kidney damage as elevated levels of albumin in urine can indicate the beginning of kidney problems. Similarly, creatinine concentration is measured within the range of 10, 50, 100, 200, and 300 mg/dL.
The ACR is calculated using the formula:
This ratio provides a standardized way to compare albumin excretion irrespective of urine concentration.
The analysis and interpretation of ACR results are categorized into three groups. An ACR of less than 30 mg/g indicates normal kidney function. An ACR between 30 and 300 mg/g suggests moderate kidney damage, necessitating further monitoring and potential intervention. An ACR of 300 mg/g or higher indicates severe kidney damage, requiring immediate medical attention.
RESULTS
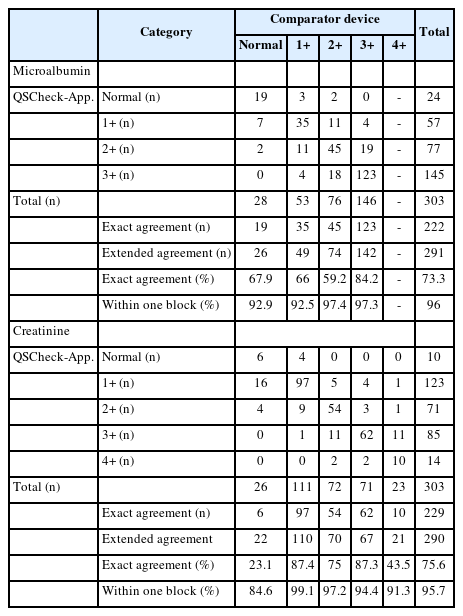
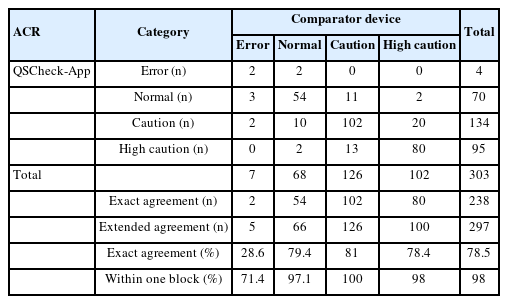
Table 3, 4 presents the results of a comparative analysis between the QSCheck-App and a comparator benchtop device across three key biomarkers: microalbumin, creatinine, and the ACR.
Microalbumin
For microalbumin testing, the EA between QSCheck-UISACR and Cybow R-50S was 67.9%, 66.0%, 59.2%, and 84.2% across the corresponding microalbumin concentration ranges, with a WOBA of 92.9%, 92.5%, 97.4%, and 97.3%, respectively, for each concentration range. The combined results showed an EA of 73.3% and a WOBA of 96.0% reflecting the aggregated data from all three hospitals (Table 3).
Creatinine
For creatinine testing, the EA between QSCheck-UISACR and Cybow R-50S was 23.1%, 87.4%, 75.0%, 87.3% and 43.5% across the corresponding creatinine concentration ranges, with WOBA values of 84.6%, 99.1%, 97.2%, 94.4% and 91.3%, respectively. The overall EA was 75.6%, and the WOBA was 95.7% (Table 3).
ACR ratio
For the ACR test results, the analysis was categorized into three ranges based on the microalbumin and creatinine concentration levels: normal, caution, and high caution. In cases where the results could not be calculated, they were classified as errors. The EA for the categories of Error, normal, caution, and high caution was 28.6%, 79.4%, 81.0%, and 78.4%, respectively. The WOBA for these categories was 71.4%, 97.1%, 100%, and 98.0%, respectively. When aggregating the data from all three hospitals, the overall EA was 78.5% and the WOBA was 98.0% (Table 4).
DISCUSSION
This study evaluated the efficacy of a novel device designed to detect and monitor albuminuria in non-hospital settings or primary care institutions without benchtop equipment. When compared to the benchtop machines in tertiary care hospitals, the device showed a concordance rate of 78.5%, despite the need for adjustments due to differences in experimental conditions. By expanding the recognition range based on differences in albuminuria in semi-quantitative tests, the concordance rate increased to 96.0%.
Albuminuria serves as both a marker for diagnosing and tracking the progression of kidney disease and an independent risk factor for cardiovascular disease [11,19]. Microalbuminuria, defined as 30–300 mg/day corresponding to 1+~2+ on semi-quantitative urine tests, is an early predictor of kidney disease and indicating potential progression even when renal function appears normal [20,21]. For patients with diabetes or hypertension, proteinuria can develop as the disease progresses. Typically, diabetic patients are tested every 6 months or annually, while hypertensive patients are not routinely tested. The significance of this device lies in its ability to be used regularly without burden, allowing for the early detection of proteinuria. Rather than focusing on absolute values, this device is valuable for tracking serial changes, enabling patients to visit healthcare providers early if proteinuria reaches a significant level, thus facilitating early evaluation for kidney disease. This early detection is crucial for increasing disease awareness and preventing or delaying disease progression through self-management. Studies in Japan have shown that in patients without diabetes, those with albuminuria had a proportional increase in the need for dialysis over 17 years of follow-up based on dipstick levels. Another study indicated that the progression of kidney function deterioration in newly diagnosed chronic kidney disease patients was proportional to the level of albuminuria [15,21].
The progression of albuminuria leading to decreased renal function is well-documented. Initially, glomerular hyperfiltration increases glomerular filtration rates, which later decline [22]. However, early diagnosis and treatment of albuminuria can improve outcomes for kidney disease and cardiovascular disease [13,19].
Traditional methods for measuring albuminuria below 300 mg/day are often insufficiently sensitive, necessitating various antibody-based methods (RIA, nephelometry, immunoturbidimetry, ELISA) to detect lower levels of albuminuria. Recently, HPLC methods have enable the detection of both immunoreactive and immunounreactive albumin, allowing for more comprehensive screening [12,14]. However, these techniques rely on expensive equipment, limiting their use in primary care settings.
The method used in this study involves a digital camera to analyze traditional urine test strips, overcoming the limitations of visual qualitative tests and providing a more cost-effective screening method. Originally developed for controlled environments with specific digital cameras, advances in digital camera technology and analysis software have enabled the use of widely accessible digital cameras for immediate analysis of urine chemistry strips. This development is part of the broader field of digital healthcare, which shows promise for high accessibility and diverse applications.
The strength of this study lies in its evaluation of the clinical utility of in vitro diagnostic devices within digital healthcare, conducted as a multi-center study to assess the device’s efficacy in various environments. The study validated the screening test’s significance by demonstrating equivalence to benchtop equipment used in hospitals.
However, there are limitations. The study did not match subjects for underlying diseases, age, or gender, and it assessed the device based on a single experiment despite controlling for other potential influences. Furthermore, the recruitment process for study participants needs to be more systematic to strengthen the accuracy of the research. Although the study compared certified medical devices, the performance differences among benchtop equipment suggest a need for equivalence evaluations with precision quantitative analyzers widely used in hospitals [23-25]. Additionally, to enhance applicability, usability assessments with diverse users and evaluations in primary care settings are necessary to address convenience. While this study assessed the clinical utility of in vitro diagnostic devices, further research is needed to improve user convenience and accuracy. Well-planned studies should validate the device’s efficacy and establish the semi-quantitative test’s performance relative to precision quantitative analyzers used in existing hospital diagnostic systems.
In conclusion, this study confirmed that the digital camera-based ACR test kit has high accuracy and concordance compared to the benchtop device used in hospitals. QSCheck-UISACR proves to be a useful tool for the early diagnosis and management of kidney disease, particularly in primary care settings. The findings suggest that this mobile diagnostic tool can improve patient accessibility and potentially reduce healthcare costs.
Notes
AUTHOR CONTRIBUTIONS
Dr. Dong-Hoon LEE had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors reviewed this manuscript and agreed to individual contributions.
Conceptualization: JR and DHL. Data curation: JR, EKP, and JHJ. Software: EKP. Formal analysis: EKP and JHJ. Project administration: DHL. Writing–original draft: DHL. Writing–review & editing: all authors.
CONFLICTS OF INTEREST
Dr. RYU has received research grants from QSTAG and serves as a consultant for QSTAG. The remaining authors declare no conflicts of interest.
FUNDING
None.
DATA AVAILABILITY
The data presented in this study are available upon reasonable request from the corresponding author.