Effects of High-Dose Intravenous Vitamin C on Colorectal Cancer Recurrence: The Retrospective Study by Medical Records for 23 Years
Article information
Abstract
Background
Among the complementary and alternative therapies used to treat cancer, studies have reported since the late 1960s that the intravenous administration of high-dose vitamin C had an anticancer effect. As the incidence of colorectal cancer has recently increased, interest in treating and preventing its recurrence has also increased. Therefore, this study investigated whether the intravenous administration of high-dose vitamin C to patients with colorectal cancer who received chemotherapy after radical resection would reduce recurrence rates.
Methods
One hundred eighty patients who were diagnosed with colorectal cancer and received chemotherapy after surgery at a university hospital in Busan for a total of 23 years from January 2000 to March 2023 were retrospectively studied. Of them, 30 were administered high-dose (1 g/kg) intravenous vitamin C injections twice weekly for more than 5 weeks in addition to standard treatments, while the remaining 150 patients who received only standard treatments were assigned to the control group. In both groups, a thorough examination was performed every year after surgery. The chi-squared test was performed to compare 5-year recurrence rates.
Results
The control group had higher recurrence and mortality rates than the experimental group; however, the differences were not statistically significant. The recurrence rate was 28.1% in the stage 2 and 3 control groups, non-significantly higher than that in the experimental group (18.8%; P>0.05).
Conclusions
There was no statistically significant difference in recurrence among patients with colorectal cancer who received chemotherapy, although the 5-year recurrence rate was decreased in the experimental versus control group.
INTRODUCTION
According to data from the Korean Statistical Information Service, the incidence of colorectal cancer in Korea is increasing (1999: 20.6/100,000 people; 2020: 54.3/100,000 people); moreover, in 2020, it was the most common cancer after thyroid and lung cancers. In 2020, 247,952 cases of cancer were diagnosed in Korea, of which colorectal cancer ranked third at 11.24% (27,877 cases), and the crude incidence rate per 100,000 people (the number of new cases in the target population during the observation period) was 54.3 [1]. Additionally, the mortality rate from colorectal cancer in 2022 was behind only lung cancer and liver cancer, accounting for 10.3% of the total cancer mortality rate [2].
High concentrations of vitamin C, which is present in fresh vegetables and fruits, were first reported by Benade et al. [3] in 1969 as having anticancer effects. Cameron and Pauling [4] found that, in patients in the final stages of various cancers who were administered intravenous vitamin C, survival was more than four times longer than that in patients not administered vitamin C. According to Riordan et al. [5], vitamin C was developed in the 1970s as an intravenous injection therapy and has shown therapeutic results as an adjuvant therapy in many clinical trials since. Riordan et al. [5] reported case studies showing that intravenous vitamin C administration reduces recurrence rates among patients with renal cell cancer, pancreatic cancer, non-Hodgkin’s lymphoma, and terminal breast cancer.
Chen et al. [6] recently revealed that vitamin C induces apoptosis, inhibits proliferation, and arrests the cell cycle in colorectal cancer and that high-dose vitamin C inhibits colorectal cancer cell growth, angiogenesis, and metastasis in xenograft mice and intraperitoneal metastasis models. In addition, Yun et al. [7] confirmed that colorectal cancer cells with mutations in KRAS or BRAF selectively die in the presence of high vitamin C concentrations.
A recent randomized open-label multicenter phase 3 study in China compared a trial group treated with Folfox at the same time as a high-dose vitamin for metastatic colorectal cancer with a comparison group treated with Folfox alone. In this study, progression-free survival, objective response rate, and overall survival all failed to improve. However, the research team is planning a future clinical study to evaluate the effects of vitamin C and bevacizumab chemotherapy [8]. Based on the research results presented above, this study retrospectively investigated the ability of high-dose vitamin C administration to reduce recurrence and mortality as an adjuvant therapy for chemotherapy in patients with colorectal cancer undergoing radical resection and chemotherapy.
METHODS
Subjects
This study was approved by the Clinical Trials and Medical Research Ethics Review Committee of the Gospel Hospital of Kosin University (institutional review board approval no. KUGH-2023-06-008).
The study involved 180 patients who were first diagnosed with colorectal cancer at Kosin University Gospel Hospital in Busan for a total of 23 years from January 2000 to March 2023 and able to be followed up for 5 years. This study included patients who received or did not receive high-dose vitamin C therapy. Patients who were diagnosed with cancer at other sites were excluded because of their past history.
Of the 180 colorectal cancer patients who received chemotherapy after diagnosis of colorectal cancer, 30 in the experimental group received vitamin C twice a week by intravenous injection (1 g/kg) for more than 5 weeks, and five times the experimental group was extracted as a group that did not receive any other treatment after the completion of chemotherapy In addition, 5:1 mating was performed based on the year of surgery, age, and sex, and the propensity score matching method was used for randomization. The subjects extracted through matching were 30 subjects in the experimental group and 150 subjects in the control group.
The patients’ medical records and pathology numbers were stored as separate files under the responsibility of the research manager, and they were coded and managed to prevent personal identification through research data.
Research-related records will be maintained for 3 years from the end of the study, and personal information among documents passed by the storage agency will be destroyed in accordance with the Personal Information Protection Act.
Procedures
This retrospective study was conducted by examining subjects’ medical records for 23 years, from January 2000 to March 2023. Colorectal cancer was defined as cancer caused by primary cancer in the colon and rectum, and there were cases where it was found while examining the large intestine due to health problems such as changes in bowel habits or bloody excrements, and were cases where it was found as a regular medical examination. Among cancers that occurred in the large intestine, cases of metastasis from other organs were excluded.
The 30 patients in the vitamin C intravenous injection experimental group received an explanation of the effects and side effects of vitamin C through counseling and signed a treatment consent form while receiving chemotherapy after surgery. When administered intravenous vitamin C, the vitamin C injection was mixed with 500 mL of physiological saline and administered within 60–80 minutes. The vitamin C used in this study was an injection solution (L-ascorbic acid) produced by UNIMED.
Among the patients receiving vitamin C, there was one case of ovarian metastasis and one case of liver metastasis, so the experimental group was 30. The control group included 150 participants from the previous list by 5:1 matching according to the year of surgery, age, and sex of the experimental group. The subjects’ age, sex, height, weight, and medical history (diabetes, high blood pressure, etc.) were retrospectively reviewed through physical examination medical records, and the primary lesion, stage, postoperative chemotherapy or radiotherapy, recurrence, and death were recorded.
Each patient’s past history was collected from their medical records, and the location of the primary cancer was extracted from the surgical record; otherwise, the name of the disease and operation were obtained from ref. Surgical and postoperative internal medicine records were referenced to determine the TNM stage of the colorectal cancer. Chemotherapy was classified based on first-line therapy and medical records recorded in the relevant department were referenced; if not, past prescription records were referenced.
Recurrence was observed at any time after surgery when it was confirmed radiologically and histologically as a metastasis of colorectal cancer (adenocarcinoma). In addition, deaths included all deaths, such as double cancer or death of old age, as well as the metastasis of colorectal cancer in the medical records.
Finally, the cancer recurrence and mortality rates of the vitamin C administration and control groups were compared, and cancer recurrence and death occurred within 5 years.
Data analysis
To investigate the association between recurrence rate, death, and intravenous vitamin C administration after cancer treatment, continuous variables are expressed as mean and standard deviation, while categorical variables are expressed as frequency and percentage. To detect differences between treatments, the t-test was used for continuous variables, the chi-square test was performed for categorical variables, and the Kaplan-Meier curve was used to confirm the recurrence and mortality rates according to the follow-up period after surgery. Recurrence and mortality rates were compared according to stage. In the data collection process, the control and experimental group setting comparison was performed by propensity score matching using the logistic regression method. SAS 9.4 (SAS Institute) was used for the analysis.
The data analysis was performed using SPSS software (version 20.0; IBM Corp.). Statistical significance was determined at values of P<0.05.
RESULTS
Subjects’ clinical characteristics
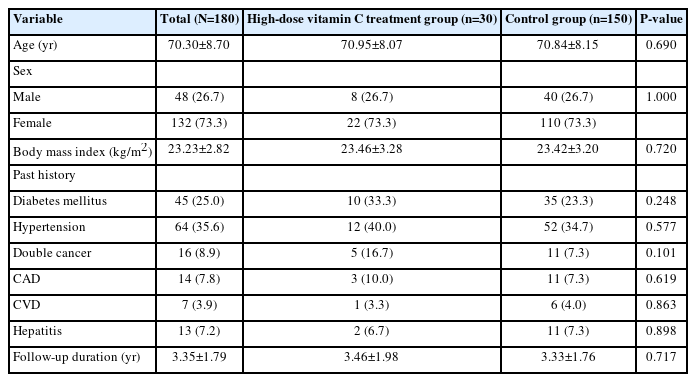
The total number of study subjects was 180; their general characteristics (age, height, weight, body mass index, etc.) are shown in Table 1. Clinical characteristics such as primary tumor and TNM stage are shown in Table 2. The analysis (Table 1) aimed to verify whether the general characteristics of the experimental and control groups were homogeneous, focusing on age, sex, body mass index, and past history. The analysis found no statistically significant intergroup difference (P>0.05), and the experimental and control group were homogeneous in terms of general characteristics. The mean follow-up period for both groups was 3.35±1.79 years for all subjects, 3.46±1.98 years for the experimental group, and 3.33±1.76 years for the control group.
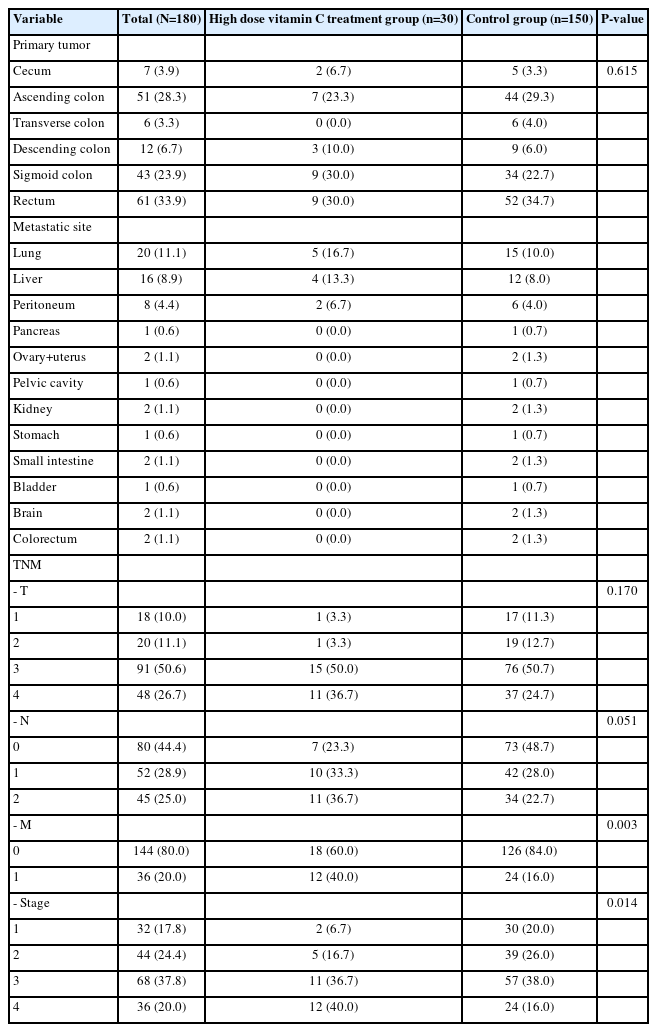
Table 2 shows the differences in primary cancer, TNM, and stage among the subjects; in terms of recurrence, the lung comprised 20 cases, while the liver comprised 16 cases. The experimental group included five cases of lung cancer and four of liver cancer, while the control group included 15 cases of lung cancer and 12 of liver cancer. In terms of TNM, the ratio of M1 was high at 40.0% (vs. control [16.0%]) in the experimental group (P<0.05), whereas in terms of stage, the ratio of the fourth stage was higher in the experimental group (40.0%) than in the control group (16.0%; P<0.05). Therefore, the statistical analysis was conducted by stage.
The statistical analysis revealed that primary cancer location and history were not important variables.
Correlation between vitamin C administration and recurrence rate
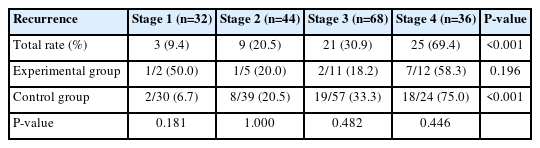
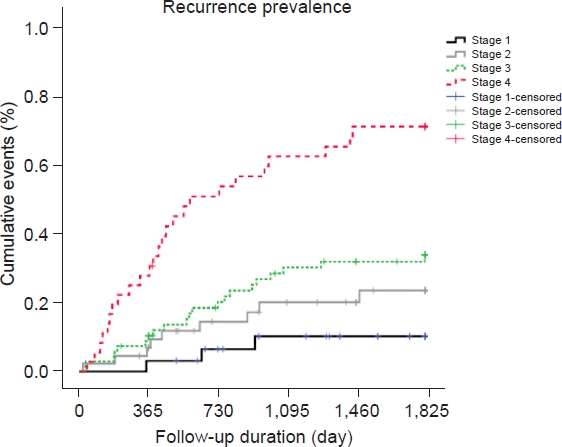
Table 3 and Fig. 1 show the differences in recurrence rates according to stage; there was a statistically significant difference in recurrence rates according to stage (P<0.05). In particular, the recurrence rate increased as the stage progressed from 1 to 4, and stage 4 had the highest recurrence rate (69.4%). According to stage, no statistically significant difference in recurrence rate was found in the experimental group (P=0.196) versus a significant difference in recurrence rates according to stage in the control group (P<0.001). In addition, no statistically significant difference was found in recurrence rates between the experimental and control groups for any stage (P>0.05); in stage 1, the experimental group had a high recurrence rate, but in stages 3 and 4, the control group showed a higher recurrence rate than the experimental group.
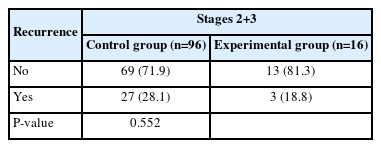
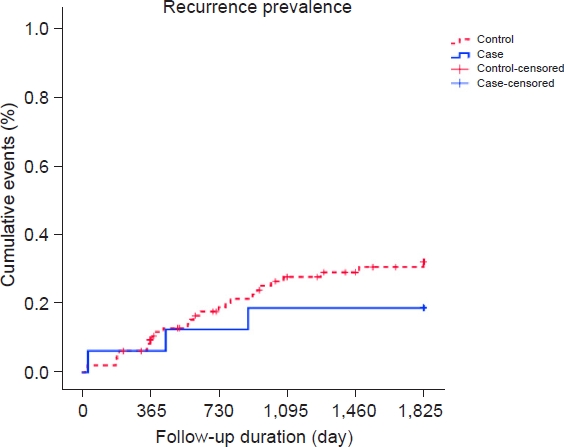
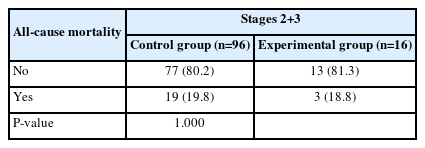
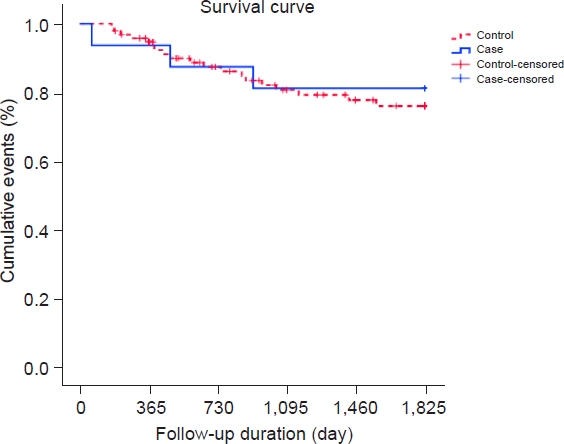
As shown in Table 4, in stages 2 and 3, the recurrence rate was higher in the control group (28.1%) than in the experimental group (18.8%), but the difference was not statistically significant (P=0.552). As shown in Fig. 2, the recurrence rate in the control group increased over time.
Correlation between vitamin C administration and mortality
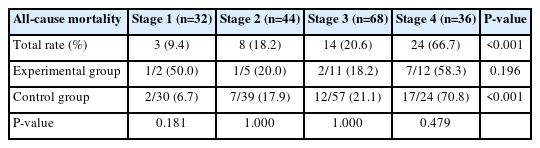
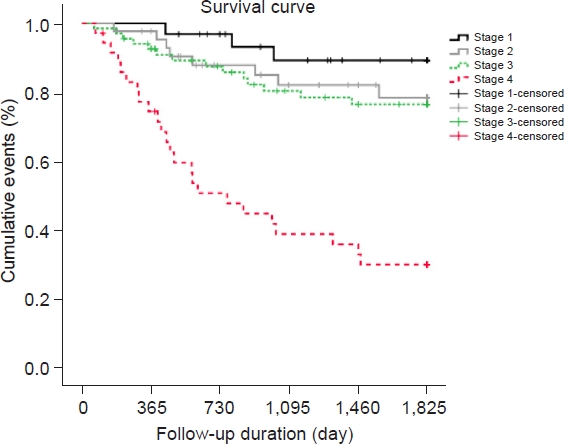
Table 5 and Fig. 3 show that there was a statistically significant difference in mortality rates according to stage (P<0.05). In particular, the mortality rate increased as the stage progressed from 1 to 4 and was highest for stage 4 (66.7%). No statistically significant difference in mortality according to stage was noted in the experimental group (P=0.196) versus a significant difference in mortality according to stage in the control group (P<0.001). No statistically significant difference was found in mortality between groups in stage 1 (P>0.05), whereas the control group had a higher mortality rate than the experimental group in stages 3 and 4.
As shown in Table 6, in stages 2 and 3, the mortality rate did not differ between the control (19.8%) and experimental (18.8%) groups (P>0.99). Moreover, no differences in mortality rate were observed between the experimental and control groups over time (Fig. 4).
DISCUSSION
Vitamin C suppresses cancer recurrence and destroys cancer cells, thereby increasing the survival rates of patients with cancer. In this study, the high-dose vitamin C group showed lower recurrence and mortality rates than the control group for colorectal cancer, but the difference was not statistically significant.
This retrospective study demonstrated that high-dose vitamin C monotherapy did not affect colorectal cancer recurrence or mortality rates. The advantage of this study is that, unlike previous studies, it included early colorectal cancer cases owing to early checkups, such as colonoscopy, which has increased since the 2010s. However, there were fewer cases of early colorectal cancer in the vitamin C administration group; therefore, the difference was not significant.
The five major vulnerabilities of cancer cells attacked by vitamin C include the redox impulse, epigenetic reprogramming, oxygen-sensing regulation, host immunity, and collagen synthesis with regard to metastasis [9]. Redox imbalance refers to vitamin C acting as an antioxidant in normal cells and a prooxidant in cancer cells, while epigenetic reprogramming refers to the reprogramming of cancer cells that changes protein translation, such as RNA transcription, unlike changes in DNA sequencing. In oxygen-sensing regulation, cancer cells inevitably become hypoxic, and among the various methods for adapting to cancer cells, there is an element that is attacked by vitamin C. Host immunity refers to several cells related to cancer cell immunity (natural killer cells, monocytes, etc.) being stimulated by vitamin C. Finally, collagen synthesis with regard to metastasis involves the process of cancer cell breakdown and synthesis of the collagen barrier, which involves vitamin C [9].
Surgery is the only treatment that can be expected to cure colorectal cancer without metastasis, and adjuvant therapy such as chemotherapy and radiation therapy can reduce the risk of recurrence, so it is being implemented in patients with stage 2 or 3 disease [10]. However, despite active surgical resection and additional treatment in patients with colorectal cancer, 30%–50% of those who undergo radical resection usually experience recurrence within 2 years [11-13].
In a survival analysis of each treatment by Kim et al. [14] in Korea, patients who underwent surgery had the highest 5-year survival rate (50.2%), followed by patients who received adjuvant therapy without surgery (14.0%), and all patients who did not receive treatment died within 5 years. However, the survival rate after radical resection of colorectal cancer is relatively high compared to that of other malignant tumors, and the high recurrence rate remains a problem. Pilipshen et al. [15] reported a recurrence rate of 44.2% after radical surgery for rectal tumors, Malcolm et al. [16] reported a recurrence rate of 27% after the radical resection of colon and rectal cancers, Adloff et al. [17] reported a recurrence rate of 31.8%, and Polk and Spratt [18] reported a recurrence rate of 31.4%.
The frequency of recurrence and metastasis increased as the stage increased; in particular, the recurrence rate was significantly higher in stage 3 than in stage 2 [19]. In addition, a comparison of the 5-year survival rates showed that stage 1 T1 was more than 95%, T2 was more than 90%, stage 2 was 70%–85%, stage 3 N1 was 50%–70%, N2 was 25%–60%, and stage 4 and distant metastasis is less than 5% [20].
High-dose vitamin therapy, one of many complementary and alternative therapies for patients with cancer, was first proposed by the renowned American chemist Pauling in the late 1970s. Cameron and Pauling [4,21] reported that patients who took 10 g of vitamin C had three to four times longer survival than those in the control group. On the other hand, Moertel et al. [22] of the Mayo Clinic argued that there was no significant anticancer effect of 10 g of oral vitamin C.
The Mayo Clinic’s research was conducted with an acceptable study design to clarify the initial observation, but it did not clearly reproduce Pauling’s research; in fact, it was much smaller and conducted in a short time. A crucial limitation of both studies (Mayo Clinic and Pauling’s) was that approximately 10 g of ascorbic acid was administered orally. In clinical practice, the concentration of vitamin C that causes cancer cell damage in vitro can be determined using intravenous injections. In addition, even if 30 g of vitamin C, which seems to be a large quantity, is given to patients with cancer, it does not reach the cancer cell toxicity-inducing plasma concentration (>200 mg/dL for density monolayers, >400 mg/dL for hollow fiber models) shown in the in vitro experiment. In other words, when the same amount of vitamin C is given, it only maintains 1/6 of the concentration in the plasma compared with that given by intravenous injection [23,24].
Although no significant difference was noted between the vitamin C and control groups in this study, a larger retrospective or prospective study could reveal more significant results. It is also meaningful to study the degree to which vitamin C administration in cancer patients reduces fatigue and improves their quality of life.
The present study had several limitations. First, the study period was 23 years; therefore, the surgery and treatment methods used throughout were heterogeneous. Second, as this was a retrospective study using previous medical records, the number of experimental patients administered high-dose vitamin C was too small (n=30). Third, it was difficult to represent all patients because the sample was composed of patients who had undergone surgery in a locally limited hospital. Fourth, in this hospital, patients with terminal colorectal cancer, that is, stage 4 colorectal cancer, receive high-dose vitamin C treatment.
Another limitation was that the effect of oral administration of vitamin C was overlooked because the subjects’ vitamin C dietary patterns could not be investigated.
High-dose vitamin injection therapy has secured considerable evidence in vitro as an alternative to prevent colon cancer metastasis and recurrence. However, clinical studies have provided insufficient evidence to support this hypothesis. Thus, future large-scale clinical studies of hyperactivity with specific chemotherapy regimens for specific cancers are warranted.
In summary, this retrospective study showed that high-dose vitamin C monotherapy does not affect recurrence or mortality rates of colorectal cancer.
Notes
AUTHOR CONTRIBUTIONS
Dr. Junjung LEE had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors reviewed this manuscript and agreed to individual contributions.
Conceptualization: JSC. Data curation: JL. Formal analysis: JL. Investigation: JL. Methodology: JSC. Validation: JSC. Writing–original draft: JL. Writing–review & editing: JSC.
CONFLICTS OF INTEREST
No existing or potential conflict of interest relevant to this article was reported.
FUNDING
None.
DATA AVAILABILITY
The data presented in this study are available upon reasonable request from the corresponding author.
ACKNOWLEDGMENTS
We would like to thank Editage (www.editage.co.kr) for English language editing. I would also like to thank Kwang-min LEE (PhD) for his advice on statistical analysis.