Effects of Intravenous Nefopam on Pain Relief in Patients with Acute Postoperative Pain: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Article information
Abstract
Background
Although intravenous nefopam has been used for opioid-sparing strategy and pain relief, randomized controlled trials (RCTs) have shown inconsistent findings.
Methods
We searched core databases, PubMed, EMBASE, and the Cochrane library for RCTs on this research question in December 2022. Standardized mean difference (SMD) and weighted mean difference (WMD) were calculated using a random-effects meta-analysis.
Results
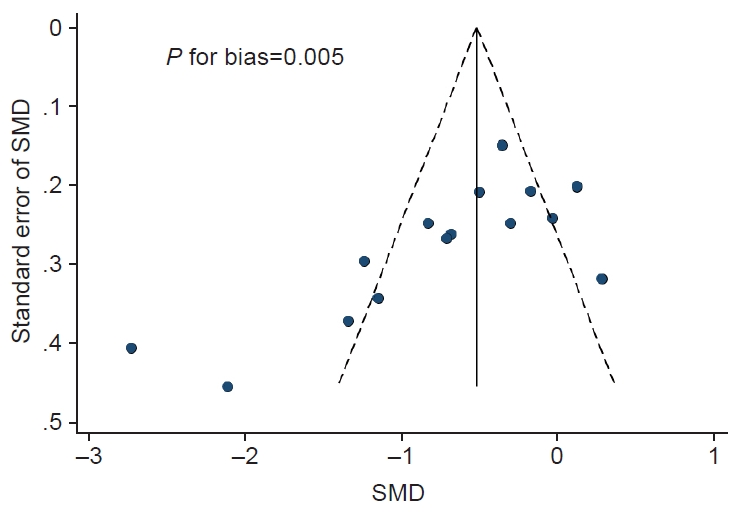
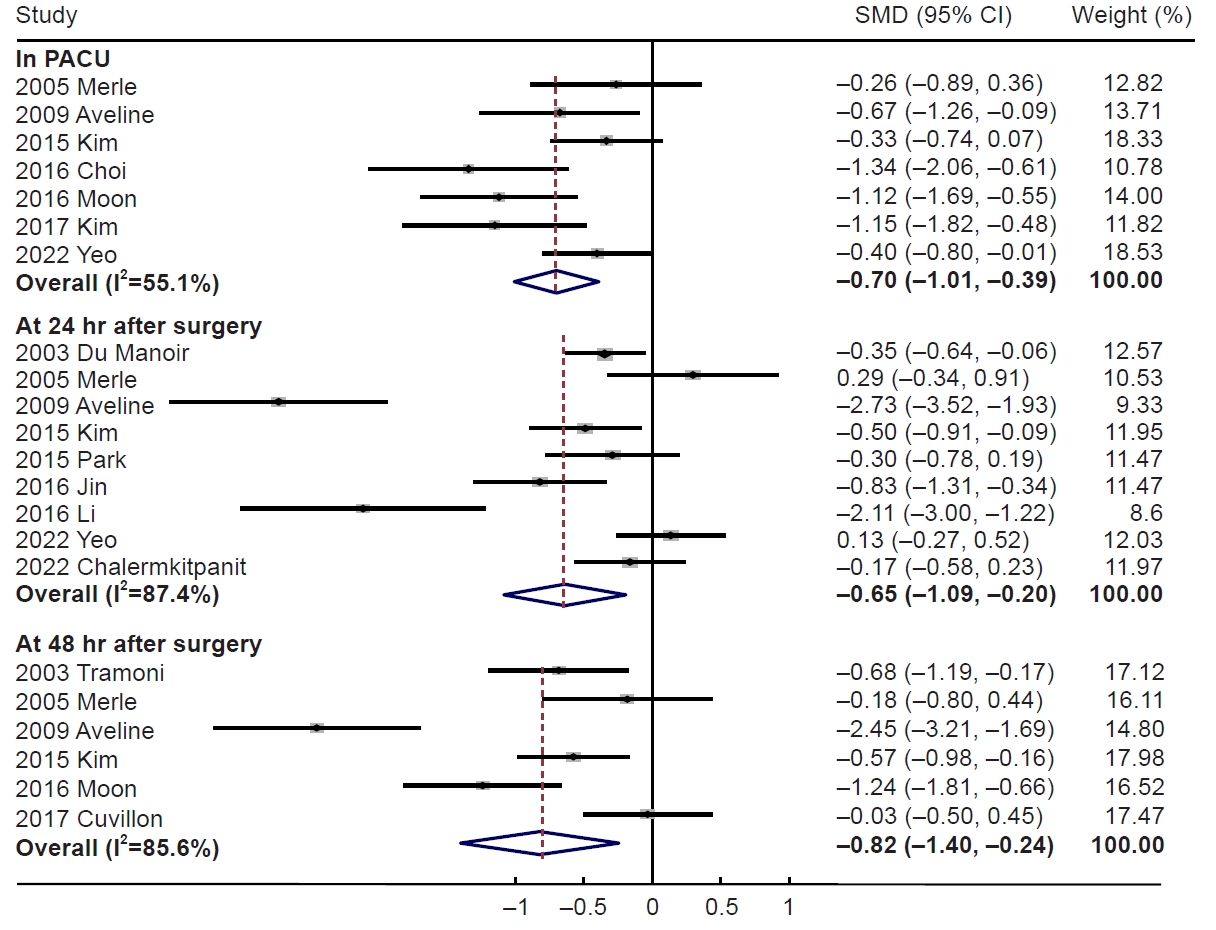
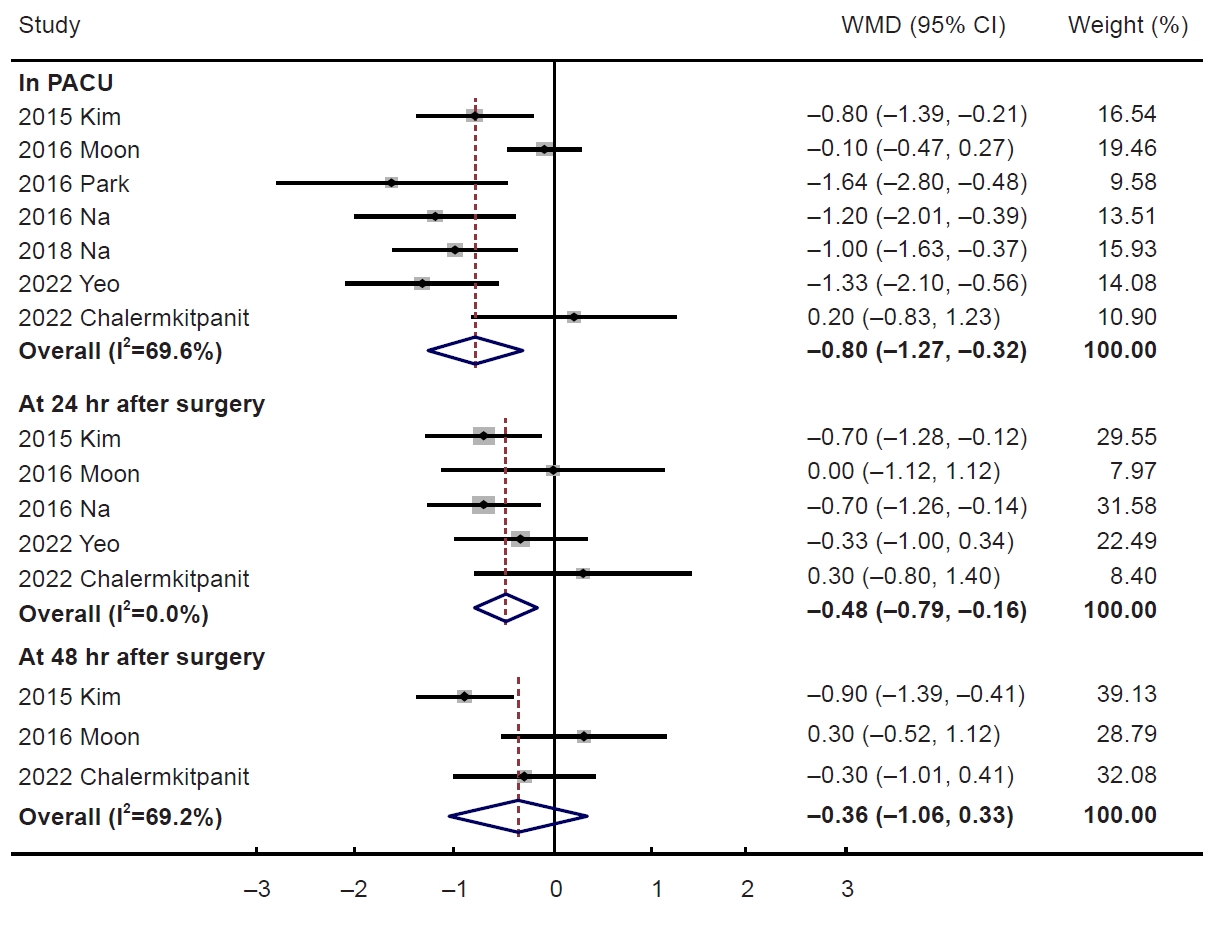
Of 708 studies identified from the databases, a total of 17 RCTs (n=1,173 patients) that met the inclusion criteria were included in the final meta-analysis. Overall, the consumption of cumulative opioid analgesics was significantly lower in the nefopam group than the control group, on arrival in the postanesthesia care unit (PACU) (SMD, −0.70; 95% confidence interval [CI], −1.01 to −0.39; I2= 55.1%; n=7), at 24 hours (SMD, −0.65; 95% CI, −1.09 to −0.20; I2=87.4%; n=9), and 48 hours (SMD, −0.82; 95% CI, −1.40 to −0.24; I2=85.6%; n=6) after surgery. It also showed a significant lower pain score, on arrival in the PACU (WMD, −0.80; 95% CI, −1.27 to −0.32; I2=69.6%; n=7) and 24 hours (WMD, −0.48; 95% CI, −0.79 to −0.16; I2=0.0%, n=5). However, publication bias was observed (asymmetrical funnel plot and P for bias=0.005).
Conclusions
Intravenous nefopam showed an opioid-sparing effect and pain relief in the management of patients with acute postoperative pain.
INTRODUCTION
Postoperative pain management is an important clinical challenge that could impact a patient’s recovery and lead to complications. Although opioids are commonly used to prevent and treat postoperative pain, their use has been limited due to their common side effects such as dependence, sedation, and respiratory depression [1]. Opioids also can paradoxically cause increased pain, which is called opioid-induced hyperalgesia [2]. To address these concerns, healthcare providers are shifting away from opioids to a supplementation of non-opioid analgesics with multiple mechanisms of action [3,4]. By using these multi-analgesic approaches, patients can achieve optimal pain relief, while reducing the total amount of opioids needed and minimizing associated side effects [3,4]. Thus, postoperative pain management became to focus on opioid-sparing approaches that incorporate a variety of analgesic agents.
For decades, nefopam, which is a non-opioid, non-steroidal, centrally acting analgesic drug and was first introduced in France in the 1970s, has been used as an alternative and supplement to opioids [5], as well as for the purpose of controlling acute painful conditions such as postoperative pain, trauma, or cancer pain [6].
Previous randomized controlled trials (RCTs) have reported inconsistent findings regarding the effect of nefopam on reduction of opioid consumption and pain relief [7-23]. Several RCTs have shown its significantly beneficial effects [7,8,10-18,20,21], whereas other RCTs did not [9,19,22,23]. In 2008, a quantitative systematic review reported that there was limited evidence that nefopam might be a useful non-opioid analgesic in the management of postoperative pain [6]. However, it only included three RCTs, and since its publication, subsequent additional RCTs on this topic have been published.
Therefore, this study aimed to investigate the effect of intravenous (IV) nefopam for opioid-sparing strategy and pain relief in patients with acute postoperative pain using a meta-analysis of RCTs.
METHODS
This meta-analysis adhered to the criteria outlined in the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [24,25].
Search strategy
We searched core electronic databases, PubMed, EMBASE, and the Cochrane library for RCTs on this research question in December 5, 2022. Literature search was conducted without language restrictions, and the keywords were (Nefopam or Acupan). Article types were confined to ‘RCTs and clinical trial’. We also reviewed the bibliographies of relevant articles to identify additional publications from previous review articles and reference lists.
Eligibility criteria
We included RCTs that fulfilled the following criteria: (1) Population, adult patients aged 18 years or older undergoing surgery under general anesthesia; (2) Intervention, perioperative administration of nefopam with opioid analgesics for postoperative pain control, IV administration via bolus injection, continuous infusion, or patient-controlled analgesia (PCA); (3) Comparisons, a placebo with opioid analgesics, or an equivalent amount of normal saline administered through the same route as the intervention group; (4) Outcome, cumulative opioid analgesics consumption and pain scores using a Numerical Rating Scale, a Visual Analogue Scale, or a Verbal Rating Scale.
Selection of studies
Two authors of this study independently reviewed and selected relevant studies based on the above mentioned selection criteria. Disagreements between the two authors were resolved by discussion. We included only full-text journal publications and excluded review articles, unpublished online clinical trial results, and abstracts. If studies overlapped, we selected the more comprehensive one. We also excluded studies that involved surgery under regional anesthesia.
Data extraction and quality assessment
In each study, we extracted the following items: author name, year of publication, number of study participants, type of surgery, type of anesthesia, nefopam regimen, postoperative analgesics, follow-up duration, and main findings.
The study quality for individual studies were assessed based on Cochrane risk of bias tool [26]. Those given a score higher than the average number of low risk of bias were considered as high-quality studies in this analysis.
Primary outcome measures
The primary outcome measures were cumulative opioid consumption on arrival in the postanesthesia care unit (PACU), at 12, 24, and 48 hours after operation, either intravenously or via IV PCA devices and resting pain scores at the same periods. Although some studies reported motion-dependent pain scores, we focused on the more commonly used resting pain scores. The secondary outcome measures included postoperative adverse events such as postoperative nausea and vomiting (PONV), confusion, sweating, tachycardia, dry mouth, dizziness, and sedation.
Statistical analysis
We calculated a pooled standardized mean difference (SMD) for cumulative opioid analgesic consumption and weighted mean difference (WMD) for pain scores with its corresponding 95% confidence intervals (CIs). When continuous results were reported as median and interquartile range (IQR), instead of mean±standard deviation, the median and IQR values were converted to the mean and standard deviation using Wan 2014 formula [27]. By incorporating effect sizes from multiple studies with different sample sizes and variances, we used a random-effects model meta-analysis based on the DerSimonian and Laird methods [26,28]. Study-wide heterogeneity was evaluated using Higgins I2 to measure the overall variance [29]. An I2 value of 50% or higher suggests that there may be substantial clinical, methodological heterogeneity [29]. Statistical analysis was performed using Stata/MP version 17.0 software package (StataCorp.).
RESULTS
Selection process
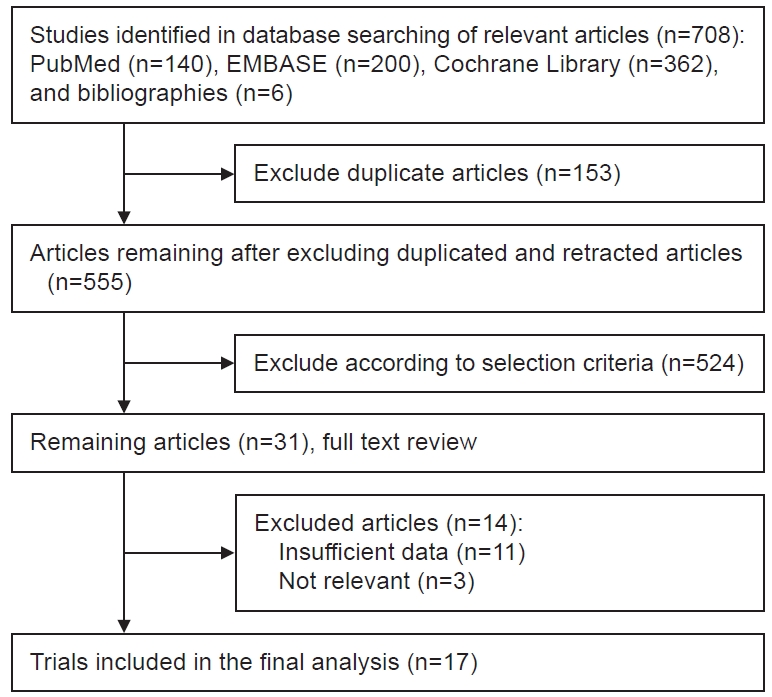
Fig. 1 shows a flow diagram for identifying relevant studies. A total of 708 articles were identified from three electronic databases and manual searches of relevant bibliographies. After removing 153 duplicates, the remaining 555 articles underwent an eligibility evaluation based on their titles and abstracts by two authors. Among them, 538 articles that did not meet the predefined selection criteria were excluded. A total of 17 articles were included in the final analysis [7-23].
General characteristics of studies
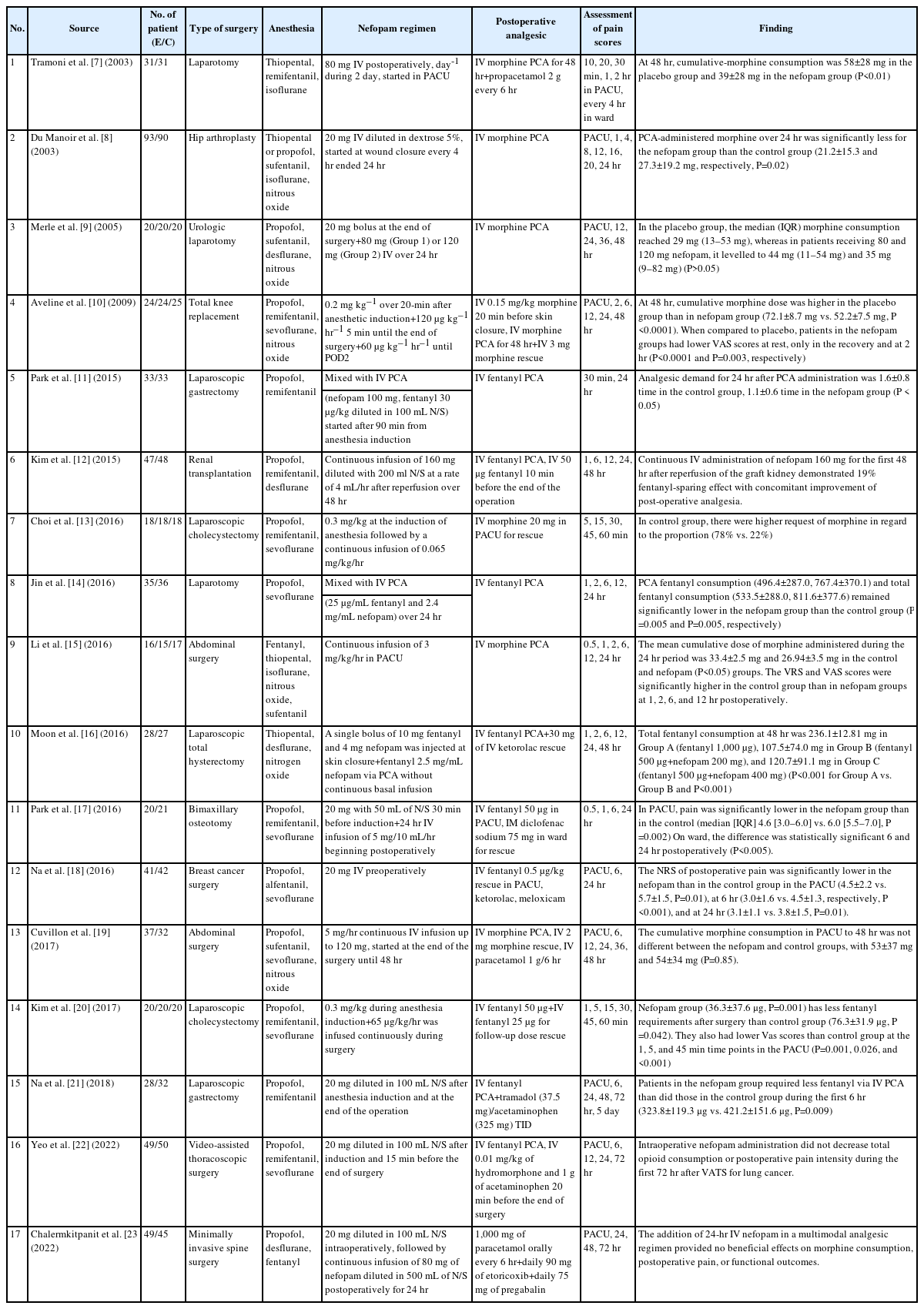
Table 1 shows general characteristics of the included studies. The eligible studies were published between 2003 and 2022, with sample sizes ranging from 31 to 183. All study participants received general anesthesia. Methods of administration for nefopam varied from study to study. The assessment of pain scores was performed at between 5 minutes and 5 days after surgery across studies.
Quality assessment
As shown in Table 2, in the methodological quality score assessment based on the Cochrane risk of bias tool, nine studies showing a low risk of bias ≥ five domains among seven domains were classified as high-quality studies [10,12-14,16-18,20,23], while the remaining eight studies having a low risk of bias in less than five domains were classified as low-quality studies.
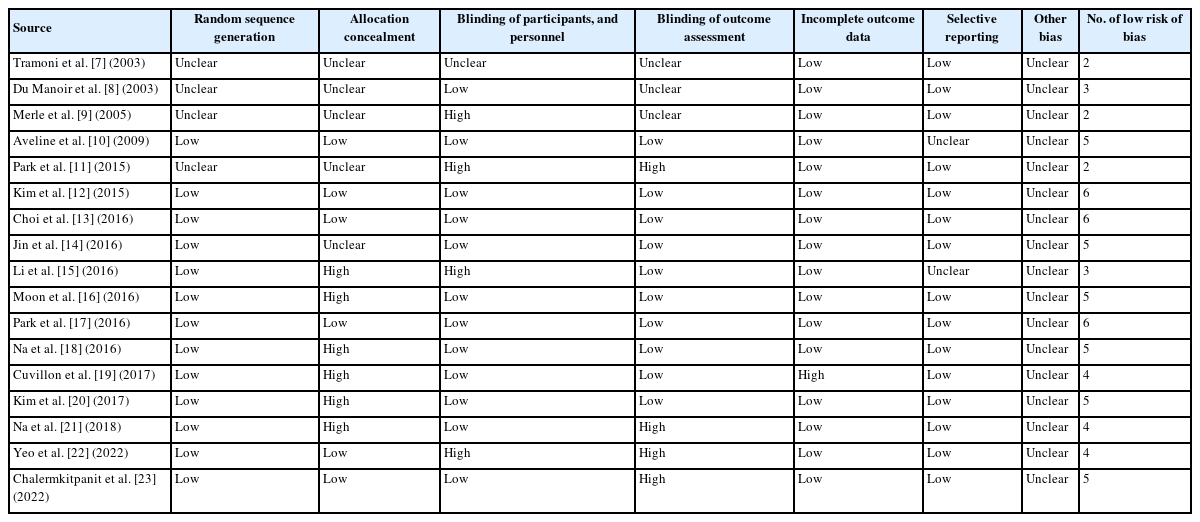
Summary of risk of bias assessment for randomized controlled trials based on the Cochrane risk of bias tool
Publication bias was observed: the Begg’s funnel plot was asymmetry, and P for bias from the Egger’s test was 0.005 (Fig. 2).
Main findings
Consumption of cumulative opioid analgesics
Of 17 RCTs, 15 reported complete data on cumulative opioid consumption in the nefopam group compared to the control group. The cumulative opioid consumption was significantly lower in the nefopam group, on arrival in the PACU (SMD, −0.70; 95% CI, −1.01 to −0.39; I2=55.1%, n=7), at 24 hours (SMD, −0.65; 95% CI, −1.09 to −0.20, I2=87.4%, n=9), and 48 hours (SMD, −0.82; 95% CI, −1.40 to −0.24; I2=85.6%, n=6) after surgery (Fig. 3). However, no significant difference was observed at 12 hours (SMD, −0.11; 95% CI, −0.40 to 0.17; I2=16.2%, n=3).
Postoperative pain scores
In a meta-analysis of seven RCTs reporting complete data on pain scores, the nefopam group showed a lower pain scores than the control group, on arrival in the PACU (WMD, −0.80; 95% CI, −1.27 to −0.32; I2=69.6%, n=7) and 24 hours (WMD, −0.48; 95% CI, −0.79 to −0.16; I2=0.0%, n=5) (Fig. 4). The pain scores at 12 hours (WMD, −0.32; 95% CI, −1.00 to 0.35; I2=73.8%, n=3) and 48 hours (WMD, −0.36; 95% CI, −1.06 to 0.33; I2=0.0%, n=3) showed no statistical differences between the two groups.
Adverse events
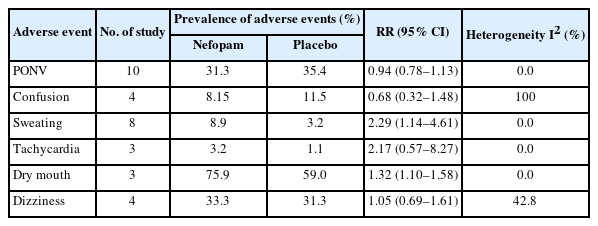
Table 3 shows the differences of adverse events between the nefopam and placebo groups. Dry mouth, PONV, and dizziness were frequently observed in both groups with the prevalence range of about 31%–76%. There was no significant difference in PONV, confusion, tachycardia, and dizziness between the two groups. However, sweating (RR, 2.29; 95% CI, 1.14 to 4.61) and dry mouth (RR, 1.32; 95% CI, 1.10 to 1.58) were significantly higher in the nefopam group compared to the placebo group.
DISCUSSION
In this meta-analysis, perioperative nefopam administration showed a significantly lower cumulative opioid consumption and pain score than that in the control group. The use of nefopam was generally safe and showed no serious adverse event: it showed higher frequency of tachycardia, sweating, and dry mouth; no significant difference in frequency of PONV and confusion was observed.
Nefopam is a non-opioid analgesic that acts centrally on the nervous system to reduce pain perception. The exact mechanism of action of nefopam has not been fully understood. However, it is known to involve multiple mechanisms. First, nefopam modulates descending pain pathways by inhibiting reuptake of triple neurotransmitters, such as norepinephrine, serotonin, or dopamine [30]. By inhibiting the reuptake of these neurotransmitters, nefopam increases their concentration in the synapses between nerve cells, which can lead to an enhancement of the descending inhibitory pain pathways. Second, by influencing the activity of voltage-gated ion channels, nefopam can affect the excitability of neurons and reduce the transmission of pain signals. Moreover, nefopam has been found to modulate glutamate transport and inhibits the activity of N-methyl-D-aspartate receptors, resulting in antihyperalgesic effects [31,32]. Finally, it has been shown to inhibit other neurotransmitters involved in pain processing, such as substance P, calcitonin gene-related peptide, and neurokinin A, which neurotransmitters are known to induce neuropathic pain by vasodilation and plasma protein extravasation [33-35]. The action of nefopam on the glutaminergic pathway has already been proven in in vitro studies, and its antiallodynic and antinociceptive effects on neuropathic pain have also been demonstrated in in vivo animal studies [35].
This meta-analysis provides a significant advantage over a previous review by including a larger number of articles and a larger sample size. Previous studies have yielded only limited evidence on the efficacy of nefopam as a non-opioid analgesic in surgical patients. However, this meta-analysis provides a high level of evidence supporting its effectiveness by pooling data from all the available studies, increasing statistical power and precision.
We adopted a uniform route of administration, specifically IV administration of nefopam, while previous reviews did not differentiate between different routes of administration [6]. The current meta-analysis showed substantial heterogeneity regarding the cumulative opioid consumption and pain score by the use of IV nefopam. It is related with a variation in dosages across individual studies. A median effective dose (ED50) of nefopam for moderate surgical pain is estimated 21.7–28 mg [36,37]. The dosage of nefopam used in studies ranged from 20 to 160 mg. Several studies reported that the estimated ED50 of nefopam as a sole agent is about 60 mg in postoperative patients who have undergone laparoscopic cholecystectomy, which dosage is higher than those commonly recommended [38]. Thus, some dosages used in the studies included in our analysis might be insufficient to effectively treat acute postoperative pain, given the varying degrees of pain associated with different types of surgeries. Substantial heterogeneity is also due to a diversity of surgical procedures, which can result in varying degrees of pain. If the pain at the time of surgery is not severe, the effect of postoperative analgesics might be underestimated. For example, laparoscopic surgery typically causes less pain than open abdominal surgery. Thus, the difference in pain scores between the nefopam and control groups might be minimal, and it might lead to a minimal difference in the dosage of opioid analgesics between groups.
In the meantime, although PONV have previously been known to be major adverse effects of perioperative administration of nefopam, our study showed no significant difference in the incidence of PONV between the two groups. Sweating was higher in the nefopam group than the control group, which is consistent with the finding of the previous systematic review [6]. Our study also found a higher incidence of dry mouth in the nefopam group compared to the control group, which contradicts the previous review [6].
Our meta-analysis has several limitations. First, as described earlier, there was substantial heterogeneity in findings, which are mainly due to clinical heterogeneity. That is, there were substantial differences in underlying diseases in the study participants, dosages and timing of nefopam, and time for pain assessment and opioid consumption. Second, most studies reported pain scores limited to the resting state, although the evaluation of dynamic pain for postoperative recovery is also important. Last, our meta-analysis showed publication bias. Thus, the effect of nefopam might be overestimated.
In conclusion, the current meta-analysis of RCTs found that over all, the use of the IV nefopam showed an opioid-sparing effect and pain relief in the management of patients with acute postoperative pain.
Notes
AUTHOR CONTRIBUTIONS
Dr. Seung-Kwon MYUNG had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors reviewed this manuscript and agreed to individual contributions.
Conceptualization: YJ and SKM. Methodology: YJ and SKM. Software: YJ and SKM. Validation: SKM. Formal analysis: YJ and SKM. Investigation: YJ, WE, and DK. Data curation: YJ and SKM. Writing-original draft: YJ and SKM. Writing-review & editing: all authors.
CONFLICTS OF INTEREST
No existing or potential conflict of interest relevant to this article was reported.
FUNDING
None.
DATA AVAILABILITY
The data presented in this study are available upon reasonable request from the corresponding author.