| Korean J Health Promot > Volume 19(4); 2019 > Article |
|
ABSTRACT
Recently, the number of obesity and diabetes mellitus have increased rapidly not only in Korea but also around the world. It is even called the new pandemic of the 21st century. In Korea, the diabetes growth rate, which exceeds the obesity growth rate, is a bigger problem. Accordingly, the simultaneous treatment of diabetes and obesity has become a global issue. In this article, we will review various obesity treatments to help diabetes remission and take a look at meaningful previous study about dietary methods for obesity. This overview includes the update of medications for obesity and the practical method for clinicians in field of obesity treatment in Korea.
REFERENCES
1. Organization for Economic Cooperation and Development (OECD). Obesity update 2017. Paris: OECD; 2017.
2. Organization for Economic Cooperation and Development (OECD). OECD health statistics 2017. Paris: OECD; 2017.
3. Korea Centers for Disease Control and Prevention (KCDC). Nation health statistics 2017. Cheongju: KCDC; 2019.
4. Korean Society for the Study of Obesity (KSSO). 2017 Obesity Fact Sheet. Seoul: KSSO; 2017.
5. Oh SW. Recent Epidemiological changes in Korean obesity. Korean J Helicobacter Up Gastrointest Res 2017;17(2):62-65.


6. The Korean Association for Survey Research. Sampling design of the 6th KNHANES and weighting adjustment of the 5th (2010–2012). Osong: Korea Centers for Disease Control and Prevention; 2013.
7. Kim YH, Han KD, Son JW, Lee SS, Oh SW, Kwon HS, et al. Data analytic process of a nationwide population-based study on obesity using the national health information database presented by the national health insurance service 2006-2015. J Obes Metab Syndr 2017;26(1):23-27.



8. Kim SG, Choi DS. The present state of diabetes mellitus in Korea. J Korean Med Assoc 2008;51(9):791-798.

9. Centers for Disease Control and Prevention (CDC). Diabetes 2017 Report Card. Atlanta: CDC; 2018.
10. Korean Diabetes Association. Diabetes Fact Sheet in Korea 2018. Seoul: Korean Diabetes Association; 2018.
11. The Economist. Heavy misconceptions, Population aged 20 years-old and above that are overweight or obese [Internet]. London: The Economist; Accessed Dec 16, 2019]. Available from: https://www.economist.com/node/21685310/mobile-frameless
12. Kim SW. Incompatibility between objective and subjective body shapes: a case in Korea. J Korean Official Stat 2017;22(4):92-120.
13. Apovian CM, Okemah J, O'Neil PM. Body weight considerations in the management of type 2 diabetes. Adv Ther 2019;36(1):44-58.




14. Dambha-Miller H, Day AJ, Strelitz J, Irving G, Griffin SJ. Behaviour change, weight loss and remission of type 2 diabetes: a community-Zbased prospective cohort study. Diabet Med 2019 09 03;[Epub ahead of print].
15. Feinmann J. Type 2 diabetes: 5000 patients to test feasibility of “remission service”. BMJ 2018;363:k5114.


16. Lean ME, Leslie WS, Barnes AC, Brosnahan N, Thom G, McCombie L, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet 2018;391(10120):541-551.


17. Hallberg SJ, McKenzie AL, Williams PT, Bhanpuri NH, Peters AL, Campbell WW, et al. Effectiveness and safety of a novel care model for the management of type 2 diabetes at 1 year: an open-label, non-randomized, controlled study. Diabetes Ther 2018;9(2):583-612.




18. Furmli S, Elmasry R, Ramos M, Fung J. Therapeutic use of intermittent fasting for people with type 2 diabetes as an alternative to insulin. BMJ Case Rep 2018;2018:bcr.



19. Wilhelmi de Toledo F, Grundler F, Bergouignan A, Drinda S, Michalsen A. Safety, health improvement and well-being during a 4 to 21-day fating period in an observational study including 1422 subjects. PLoS One 2019;14(1):e0209353.



20. Taylor R, Al-Mrabeh A, Zhyzhneuskaya S, Peters C, Barnes AC, Aribisala BS, Hollingsworth KG, et al. Remission of human type 2 diabetes requires decrease in liver and pancreas fat content but is dependent upon capacity for β cell recovery. Cell Metab 2018;28(4):667.


21. Lean M, Hankey C. Keeping it off: the challenge of weight loss maintenance. Lancet Diabetes Endocrinol 2018;6(9):681-683.


22. Lean MEJ, Leslie WS, Barnes AC, Brosnahan N, Thom G, McCombie L, et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol 2019;7(5):344-355.


24. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359(15):1577-1589.


25. de Boer IH, Rue TC, Cleary PA, Lachin JM, Molitch ME, Steffes MW, et al. Long-term renal outcomes of patients with type 1 diabetes mellitus and microalbuminuria: an analysis of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications cohort. Arch Intern Med 2011;171(5):412-420.



26. Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353(25):2643-2653.



27. Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular outcomes in type 1 diabetes: the DCCT/EDIC study 30-year follow-up. Diabetes Care 2016;39(5):686-693.




28. Athinarayanan SJ, Adams RN, Hallberg SJ, McKenzie AL, Bhanpuri NH, Campbell WW, et al. Long-term effects of a novel continuous remote care intervention including nutritional ketosis for the management of type 2 diabetes: a 2-year non-randomized clinical trial. Front Endocrinol (Lausanne) 2019;10:348.



29. Bhanpuri NH, Hallberg SJ, Williams PT, McKenzie AL, Ballard KD, Campbell WW, et al. Cardiovascular disease risk factor responses to a type 2 diabetes care model including nutritional ketosis induced by sustained carbohydrate restriction at 1 year: an open label, non-randomized, controlled study. Cardiovasc Diabetol 2018;17(1):56.




30. Frias JP, Nauck MA, Van J, Kutner ME, Cui X, Benson C, et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet 2018;392(10160):2180-2193.


31. Sjöström L. Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. J Intern Med 2013;273(3):219-234.



32. Lusting RH. Fat chance. New York: Penguin Group (USA) LLC; 2012.
34. Lustig RH, Schmidt LA, Brindis CD. Public health: the toxic truth about sugar. Nature 2012;482(7383):27-29.



35. van Buul VJ, Tappy L, Brouns FJ. Misconceptions about fructosecontaining sugars and their role in the obesity epidemic. Nutr Res Rev 2014;27(1):119-113.



36. Lustig RH, Mulligan K, Noworolski SM, Tai VW, Wen MJ, Erkin-Cakmak A, et al. Isocaloric fructose restriction and metabolic improvement in children with obesity and metabolic syndrome. Obesity (Silver Spring) 2016;24(2):453-460.




37. Williams KJ, Wu X. Imbalanced insulin action in chronic over nutrition: clinical harm, molecular mechanisms, and a way forward. Atherosclerosis 2016;247:225-282.


38. K Hall KD, Sacks G, Chandramohan D, Chow CC, Wang YC, Gortmaker SL, et al. Quantification of the effect of energy imbalance on bodyweight. Lancet 2011;378(9793):826-837.


39. Walmart. Prices and nutritional content of foods [Internet]. Philadelphia: Walmart; 2014;Accessed Nov 6, 2014]. Available from: http://www.walmart.com
40. Stroebele N, Hill JO, Willich SN. Identifying the energy gap in the German population using results from representative national health surveys (1985-2002). Public Health Nutr 2011;14(1):44-48.


41. Ogden CL, Fryar CD, Carroll MD, Flegal KM. Mean body weight, height, and body mass index, United States 1960-2002. Adv Data 2004;(347):1-17.
42. Champagne CM, Han H, Bajpeyi S, Rood J, Johnson WD, Lammi-Keefe CJ, et al. Day-to-day variation in food intake and energy expenditure in healthy women: the Dietitian II Study. J Acad Nutr Diet 2013;113(11):1532-1538.


43. Fildes A, Charlton J, Rudisill C, Littlejohns P, Prevost AT, Gulliford MC. Probability of an obese person attaining normal body weight: cohort study using electronic health records. Am J Public Health 2015;105(9):e54-e59.



44. Sumithran P, Prendergast LA, Delbridge E, Purcell K, Shulkes A, Kriketos A, et al. Long term persistence of hormonal adaptiation to weight loss. N Engl J Med 2011;365(17):1597-1604.


45. Fothergill E, Guo J, Howard L, Kerns JC, Knuth ND, Brychta R, et al. Persistent metabolic adaptation 6 years after “the biggest Loser” competition. Obesity (Silver Spring) 2016;24(8):1612-1619.



46. Johansson K, Neovius M, Hemmingsson E. Effects of anti-obesity drugs, diet, and exercise on weight-loss maintenance after a very-low-calorie diet or low-calorie diet: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr 2014;99(1):14-23.



47. The National Weight Control Registry (NWCR). NWCR facts [Internet]. Providence: NWCR; Accessed Dec 16, 2019]. Available from: http://www.nwcr.ws/Research/default.htm
48. Thomas JG, Bond DS, Phelan S, Hill JO, Wing RR. Weightloss maintenance for 10 years in the National Weight Control Registry. Am J Prev Med 2014;46(1):17-23.


49. Soleymani T, Daniel S, Garvey WT. Weight maintenance: challenges, tools and strategies for primary care physicians. Obes Rev 2016;17(1):81-93.




50. Gilis-Januszewska A, Barengo NC, Lindström J, Wojtowicz E, Acosta T, Tuomilehto J, et al. Predictors of long term weight loss maintenance in patients at high risk of type 2 diabetes participating in a lifestyle intervention program in primary health care: The DE-PLAN study. PLoS One 2018;13(3):e0194589.



51. Snorgaard O, Poulsen GM, Andersen HK, Astrup A. Systematic review and meta-analysis of dietary carbohydrate restriction in patients with type 2 diabetes. BMJ Open Diabetes Res Care 2017;5(1):e000354.



52. van Wyk HJ, Davis RE, Davies JS. A critical review of low-carbohydrate diets in people with type 2 diabetes. Diabet Med 2016;33(2):148-157.



53. Tay J, Thompson CH, Luscombe-Marsh ND, Wycherley TP, Noakes M, Buckley JD, et al. Effects of an energy-restricted low-carbohydrate, high unsaturated fat/low saturated fat diet versus a high-carbohydrate, low-fat diet in type 2 diabetes: a 2-year randomized clinical trial. Diabetes Obes Metab 2018;20(4):858-871.



54. van Zuuren EJ, Fedorowicz Z, Kuijpers T, Pijl H. Effects of low-carbohydrate-compared with low-fat-diet interventions on metabolic control in people with type 2 diabetes: a systematic review including GRADE assessments. Am J Clin Nutr 2018;108(2):300-331.


55. Naude CE, Schoonees A, Senekal M, Young T, Garner P, Volmink J. Low carbohydrate versus isoenergetic balanced diets for reducing weight and cardiovascular risk: a systematic review and meta-analysis. PLoS One 2014;9(7):e100652.



56. Gill S, Panda S. A smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell Metab 2015;22(5):789-798.



57. Gabel K, Hoddy KK, Haggerty N, Song J, Kroeger CM, Trepanowski JF, et al. Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: a pilot study. Nutr Healthy Aging 2018;4(4):345-353.



58. Antoni R, Robertson TM, Robertson MD, Johnston JD. A pilot feasibility study exploring the effects of a moderate time-restricted feeding intervention on energy intake, adiposity and metabolic physiology in free-living human subjects. J Nutr Sci 2018;7:E22.

59. Hutchison AT, Regmi P, Manoogian ENC, Fleischer JG, Wittert GA, Panda S, et al. Time-restricted feeding improves glucose tolerance in men at risk for type 2 diabetes: a randomized crossover trial. Obesity (Silver Spring) 2019;27(5):724-732.



60. Wilkinson MJ, Manoogian ENC, Zadourian A, Lo H, Fakhouri S, Shoghi A, et al. Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Cell Metab 2019 12 2;[Epub ahead of print].
61. Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab 2018;27(6):1212-1221.e3.



62. Harris L, Hamilton S, Azevedo LB, Olajide J, De Brún C, Waller G, et al. Intermittent fasting interventions for treatment of overweight and obesity in adults: a systematic review and meta-analysis. JBI Database System Rev Implement Rep 2018;16(2):507-547.


63. Gardner CD, Kiazand A, Alhassan S, Kim S, Stafford RS, Balise RR, et al. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z weight loss study: a randomized trial. JAMA 2007;297(9):969-977.


64. Johnston BC, Kanters S, Bandayrel K, Wu P, Naji F, Siemieniuk RA, et al. Comparison of weight loss among named diet programs in overweight and obese adults: a meta-analysis. JAMA 2014;312(9):923-933.


65. Gardner CD, Trepanowski JF, Del Gobbo LC, Hauser ME, Rigdon J, Ioannidis JPA, et al. Effect of low-fat vs low-carbohydrate diet on 12-month weight loss in overweight adults and the association with genotype pattern or insulin secretion: the DIETFITS randomized clinical trial. JAMA 2018;319(7):667-679.



66. Dehghan M, Mente A, Zhang X, Swaminathan S, Li W, Mohan V, et al. Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): a prospective cohort study. Lancet 2017;390(10107):2050-2062.

67. Seidelmann SB, Claggett B, Cheng S, Henglin M, Shah A, Steffen LM, et al. Dietary carbohydrate intake and mortality: a prospective cohort study and meta-analysis. Lancet Public Health 2018;3(9):e419-e428.



68. huang X, Zhang S, Zhou H, Du Z, Liao X. U-shaped relationship between carbohydrate intake proportion and incident atrial fibrillation. J Am Coll Cardiol 2019;73(9):4.

69. Reynolds A, Mann J, Cummings J, Winter N, Mete E, Te Morenga L. Carbohydrate quality and human health: a series of systematic reviews and meta-analyses. Lancet 2019;393(10170):434-445.


70. Purcell K, Sumithran P, Prendergast LA, Bouniu CJ, Delbridge E, Proietto J. The effect of rate of weight loss on long-term weight management: a randomised controlled trial. Lancet Diabetes Endocrinol 2014;2(12):954-962.


71. Nackers LM, Ross KM, Perri MG. The association between rate of initial weight loss and long-term success in obesity treatment: does slow and steady win the race? Int J Behav Med 2010;17(3):161-167.




72. Unick JL, Neiberg RH, Hogan PE, Cheskin LJ, Dutton GR, Jeffery R, et al. Weight change in the first 2 months of a lifestyle intervention predicts weight changes 8 years later. Obesity (Silver Spring) 2015;23(7):1353-1356.



73. Sievert K, Hussain SM, Page MJ, Wang Y, Hughes HJ, Malek M, et al. Effect of breakfast on weight and energy intake: systematic review and meta-analysis of randomised controlled trials. BMJ 2019;364:l42.



75. McClernon FJ, Yancy WS Jr, Eberstein JA, Atkins RC, Westman EC. The effects of a low-carbohydrate ketogenic diet and a low-fat diet on mood, hunger, and other self-reported symptoms. Obesity (Silver Spring) 2007;15(1):182-187.


76. Hendricks EJ, Rothman RB, Greenway FL. How physician obesity specialists use drugs to treat obesity. Obesity (Silver Spring) 2009;17(9):1730-1735.



77. Thomas CE, Mauer EA, Shukla AP, Rathi S, Aronne LJ. Low adoption of weight loss medications: A comparison of prescribing patterns of antiobesity pharmacotherapies and SGLT2s. Obesity (Silver Spring) 2016;24(9):1955-1961.


78. Novo Nordisk. Novo Nordisk Annual Report 2018. Bagsværd: Novo Nordisk; 2019.
79. Lee SJ. Serious Abuse of Psychotropic Anti-Obesity Drugs; Suspect of over-prescription by doctor [Internet]. Seoul: MedicalTimes; 2013;Accessed MON DAY, YEAR]. Avaliable from: http://www.medicaltimes.com/Users/News/NewsView.html?ID=1085905
80. O'Neil PM, Birkenfeld AL, McGowan B, Mosenzon O, Pedersen SD, Wharton S, et al. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss inpatientswithobesity: a randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial. Lancet 2018;392(10148):637-649.


81. Mancini MC, de Melo ME. The burden of obesity in the current world and the new treatments available: focus on liraglutide 3.0 mg. Diabetol Metab Syndr 2017;9:44.




82. Munro JF, MacCuish AC, Wilson EM, Duncan LJ. Comparison of continuous and intermittent anorectic therapy in obesity. Br Med J 1968;1(5588):352-354.



83. Aronne LJ, Wadden TA, Peterson C, Winslow D, Odeh S, Gadde KM. Evaluation of phentermine and topiramate versus phentermine/topiramate extended-release in obese adults. Obesity (Silver Spring) 2013;21(11):2163-2171.



84. Garvey WT, Ryan DH, Bohannon NJ, Kushner RF, Rueger M, Dvorak RV, et al. Weight-loss therapy in type 2 diabetes: effects of phentermine and topiramate extended release. Diabetes Care 2014;37(12):3309-3316.



85. Hollander P, Bays HE, Rosenstock J, Frustaci ME, Fung A, Vercruysse F, et al. Coadministration of canagliflozin and phentermine for weight management in overweight and obese individuals without diabetes: a randomized clinical trial. Diabetes Care 2017;40(5):632-639.



86. Smith SR, Garvey WT, Greenway FL, Zhou S, Fain R, Pilson R, et al. Coadministration of lorcaserin and phentermine for weight management: a 12-week, randomized, pilot safety study. Obesity (Silver Spring) 2017;25(5):857-865.




87. Moldovan CP, Weldon AJ, Daher NS, Schneider LE, Bellinger DL, Berk LS, et al. Effects of a meal replacement system alone or in combination with phentermine on weight loss and food cravings. Obesity (Silver Spring) 2016;24(11):2344-2350.


88. Lewis KH, Fischer H, Ard J, Barton L, Bessesen DH, Daley MF, et al. Safety and effectiveness of longer-term phentermine use: clinical outcomes from an electronic health record cohort. Obesity (Silver Spring) 2019;27(4):591-602.



89. Onakpoya IJ, Heneghan CJ, Aronson JK. Post-marketing withdrawal of anti-obesity medicinal products because of adverse drug reactions: a systematic review. BMC Med 2016;14(1):191.




90. Heck AM, Yanovski JA, Calis KA. Orlistat, a new lipase inhibitor for the management of obesity. Pharmacotherapy 2000;20(3):270-279.



91. Torgerson JS, Hauptman J, Boldrin MN, Sjöström L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 2004;27(1):155-161.

92. Patel DK, Stanford FC. Safety and tolerability of new-generation anti-obesity medications: a narrative review. Postgrad Med 2018;130(2):173-182.



93. Weir MA, Beyea MM, Gomes T, Juurlink DN, Mamdani M, Blake PG, et al. Orlistat and acute kidney injury: an analysis of 953 patients. Arch Intern Med 2011;171(7):703-704.


94. Kopelman P, Groot Gde H, Rissanen A, Rossner S, Toubro S, Palmer R, et al. Weight loss, HbA1c reduction, and tolerability of cetilistat in a randomized, placebo-controlled phase 2 trial in obese diabetics: comparison with orlistat (Xenical). Obesity (Silver Spring) 2010;18(1):108-115.



95. Bohula EA, Wiviott SD, McGuire DK, Inzucchi SE, Kuder J, Im K, et al. Cardiovascular safety of Lorcaserin in overweight or obese patients. N Engl J Med 2018;379(12):1107-1117.


96. Bohula EA, Scirica BM, Fanola C, Inzucchi SE, Keech A, McGuire DK, et al. Design and rationale for the cardiovascular and metabolic effects of lorcaserin in overweight and obese patients-thrombolysis in myocardial infarction 61 (CAMELLIATIMI 61) trial. Am Heart J 2018;202:39-48.


97. Farr OM, Upadhyay J, Gavrieli A, Camp M, Spyrou N, Kaye H, et al. Lorcaserin administration decreases activation of brain centers in response to food cues and these emotion- and salience-related changes correlate with weight loss effects: a 4-week-long randomized, placebo-controlled, double-blind clinical trial. Diabetes 2016;65(10):2943-2953.




98. Allison DB, Gadde KM, Garvey WT, Peterson CA, Schwiers ML, Najarian T, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring) 2012;20(2):330-342.




99. Gadde KM, Allison DB, Ryan DH, Peterson CA, Troupin B, Schwiers ML, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet 2011;377(9774):1341-1352.


100. Garvey WT, Ryan DH, Look M, Gadde KM, Allison DB, Peterson CA, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr 2012;95(2):297-308.

101. Winslow DH, Bowden CH, DiDonato KP, McCullough PA. A randomized, double-blind, placebo-controlled study of anoral, extended-release formulation of phentermine/topiramate for the treatment of obstructive sleep apnea in obese adults. Sleep 2012;35(11):1529-1539.



102. Guerdjikova AI, Williams S, Blom TJ, Mori N, McElroy SL. Combination phentermine-topiramate extended release for the treatment of binge eating disorder: an open-label, Prospective Study. Innov Clin Neurosci 2018;15(5-6):17-21.
103. Ritchey ME, Harding A, Hunter S, Peterson C, Sager PT, Kowey PR, et al. Cardiovascular safety during and after use of phentermine and topiramate. J Clin Endocrinol Metab 2019;104(2):513-522.




104. Ard JD, Beavers DP, Hale E, Miller G, McNatt S, Fernandez A. Use of phentermine-topiramate extended release in combination with sleeve gastrectomy in patients with BMI 50 kg/m2 or more. Surg Obes Relat Dis 2019;15(7):1039-1043.


105. Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med 2015;373(1):11-22.


106. Wadden TA, Hollander P, Klein S, Niswender K, Woo V, Hale PM, et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE maintenance randomized study. Int J Obes (Lond) 2013;37(11):1443-1451.



107. Davies MJ, Bergenstal R, Bode B, Kushner RF, Lewin A, Skjøth TV, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA 2015;314(7):687-699.


108. Blackman A, Foster GD, Zammit G, Rosenberg R, Aronne L, Wadden T, et al. Effect of liraglutide 3.0 mg in individuals with obesity and moderate or severe obstructive sleep apnea: the SCALE Sleep Apnea randomized clinical trial. Int J Obes (Lond) 2016;40(8):1310-1319.




109. Hegedüs L, Sherman SI, Tuttle RM, von Scholten BJ, Rasmussen S, Karsbøl JD, et al. No evidence of increase in calcitonin concentrations or development of C-cell malignancy in response to Liraglutide for up to 5 years in the LEADER trial. Diabetes Care 2018;41(3):620-622.



110. Fujioka K, O'Neil PM, Davies M, Greenway F, C W Lau D, Claudius B, et al. Early weight loss with liraglutide 30 mg predicts 1-year weight loss and is associated with improvements in clinical markers. Obesity (Silver Spring) 2016;24(11):2278-2288.




111. Iepsen EW, Lundgren J, Dirksen C, Jensen JE, Pedersen O, Hansen T, et al. Treatment with a GLP-1 receptor agonist diminishes the decrease in free plasma leptin during maintenance of weight loss. Int J Obes (Lond) 2015;39(5):834-841.




112. de Boer SA, Lefrandt JD, Petersen JF, Boersma HH, Mulder DJ, Hoogenberg K. The effects of GLP-1 analogues in obese, insulin-using type 2 diabetes in relation to eating behaviour. Int J Clin Pharm 2016;38(1):144-151.




113. Sharma A, Ambrosy AP, DeVore AD, Margulies KB, McNulty SE, Mentz RJ, et al. Liraglutide and weight loss among patients with advanced heart failure and a reduced ejection fraction: Insights from the FIGHT trial. ESC Heart Fail 2018;5(6):1035-1043.


114. Larsen JR, Vedtofte L, Jakobsen MSL, Jespersen HR, Jakobsen MI, Svensson CK, et al. Effect of liraglutide treatment on prediabetes and overweight or obesity in clozapine- or olanzapine-treated patients with schizophrenia spectrum disorder: a randomized clinical trial. JAMA Psychiatry 2017;74(7):719-728.



115. Simonds SE, Pryor JT, Koegler FH, Buch-Rasmussen AS, Kelly LE, Grove KL, et al. Determining the effects of combined liraglutide and phentermine on metabolic parameters, blood pressure and heart rate in lean and obese male mice. Diabetes 2019;68(4):683-695.



116. Dunn E. A retrospective comparative analysis on the effectiveness of pharmacologic weight loss [dissertation]. Lexington: University of Kentucky College of Nursing; 2018;English.
117. Greig SL, Keating GM. Naltrexone ER/bupropion ER: a review in obesity management. Drugs 2015;75(11):1269-1280.



118. Greenway FL, Fujioka K, Plodkowski RA, Mudaliar S, Guttadauria M, Erickson J, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2010;376(9741):595-605.


119. Apovian CM, Aronne L, Rubino D, Still C, Wyatt H, Burns C, et al. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II). Obesity (Silver Spring) 2013;21(5):935-943.




120. Hollander P, Gupta AK, Plodkowski R, Greenway F, Bays H, Burns C, et al. Effects of naltrexone sustained-release/bupropion sustained release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care 2013;36(12):4022-4029.




121. Wadden TA, Foreyt JP, Foster GD, Hill JO, Klein S, O'Neil PM, et al. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity (Silver Spring) 2011;19(1):110-120.




122. Srivastava G, Apovian C. Future pharmacotherapy for obesity: new anti-obesity drugs on the horizon. Curr Obes Rep 2018;7(2):147-161.



123. Gaich G, Chien JY, Fu H, Glass LC, Deeg MA, Holland WL, et al. The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab 2013;18(3):333-340.


124. Azegami T, uki Y, Sawada S, Mejima M, Ishige K, Akiyoshi K, et al. Nanogel-based nasal ghrelin vaccine prevents obesity. Mucosal Immunol 2017;10(5):1351-1360.



Figure 3
Remission of type 2 diabetes after primary care for weight management shown by DiRECT study.16) DiRECT, Diabetes Remission Clinical Trial; CI, confidence interval.

Figure 4
Long-term effects of a novel continuous remote care intervention including nutritional ketosis for the management of type 2 diabetes shown by Low Carb High Fat (LCHF) study for 2 years.17) HbA1c, hemoglobin A1c.

Figure 5
Association between estimated percentage energy from fat versus carbohydrate and total mortality shown by Prospective Urban Rural Epidemiology (PURE) study.66) HR, hazard ratio.

Figure 6
(A, B) U-shaped association between percentage of energy from carbohydrate and all-cause mortality in the Atherosclerosis Risk in Communities (ARIC) (line) and Prospective Urban Rural Epidemiology (PURE) (dotted line) cohort studies.67)

Figure 7
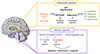
Mechanism of anti-obesity medication about homeostatic appetite and hedonic appetite.81) ARC, arcuate nucleus; GABA, gamma-aminobutyric acid.
-
METRICS

-
- 3 Crossref
- 0 Scopus
- 2,590 View
- 8 Download
- Related articles







 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print